ISSN: 2206-7418
Nanotheranostics 2025; 9(3):315-334. doi:10.7150/ntno.118766 This issue Cite
Review
Synergestic Protein-Green synthesized Nanoparticles Nanosystems: A Sustainable and Safe Approach for Cancer Theranostics
Computational Biology and Bioinformatics Lab, Department of Bioscience and Engineering, National Institute of Technology Calicut, Kozhikode, Kerala, India, 673601.
Received 2025-6-3; Accepted 2025-10-9; Published 2025-10-24
Abstract
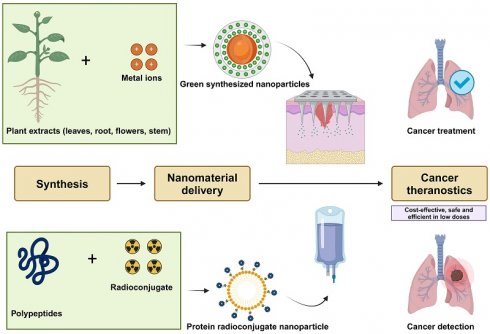
Rationale: Cancer theranostics is an evolving field focused on reducing mortality and providing safer treatment options for complete remission. The rising incidence of cancer and increased mortality linked to frequent hospital visits highlight the need for nature-based remedies as promising alternatives. Nanotechnology has contributed to cancer treatment by offering effective anti-cancer and antimicrobial solutions, with a current emphasis on developing safe, easily synthesized nanomaterials using natural sources and green synthesis methods.
Methods: This review explores the synergistic use of protein-based nanomaterials and green-synthesized nanoparticles in cancer theranostics. Sources of protein-based nanomaterials include human serum albumin, gliadin, DNA, peptides, collagen, bacteria, and soy protein. Green-synthesized nanoparticles discussed include gold, silver, copper, zinc, and magnesium. The approach involves evaluating the stability, biocompatibility, and therapeutic potential of these nanosystems based on existing experimental findings.
Results: Protein-based nanomaterials and green-synthesized nanoparticles demonstrate synergistic effects that enhance their stability and efficacy in cancer theranostics. These nanosystems offer anti-cancer activity along with additional functional properties resulting from their synergistic composition. Furthermore, they are environmentally friendly and non-toxic. Despite their promise, the literature reveals a gap in studies investigating these hybrid nanosystems, particularly regarding in vivo evaluations.
Conclusions: Synergistic protein-green synthesized nanoparticle nanosystems hold significant promise for cancer theranostics due to their enhanced therapeutic properties and environmental safety. However, additional in vivo studies are crucial to fully establish their efficacy. Future research should leverage emerging technologies to accelerate the development and testing of stable nanosystems for clinical application.
Keywords: Cancer theranostics, Nanomaterials, Nanoparticles, Nanostructures, Nanosystems
Introduction
Cancer is an umbrella term that refers to the proliferation and unrestricted development of abnormal cells within various body parts and systems, including the blood, skin, digestive, urinary, respiratory, skeletal, reproductive, and neural systems [1,2]. Cancer remains one of the most lethal diseases in the world, with new cases increasing year after year [3]. International Agency for Research on Cancer reports estimates that one in every four deaths due to non-communicable diseases is caused due to cancer and an estimated of 20 million new cases were reported in 2022 alone [4,5]. The report also estimates that approximately half of the diagnosed cases would die, making the mortality rate almost 50 percent [6]. The estimates are expected to be much higher as there are data insufficiencies due to lack of data from some countries, especially from low income and middle-income countries; data inconsistencies during data collection are another reason [6]. Research about tumor biology along with advancements in diagnostic techniques and anti-cancer therapies has contributed a significant 29% reduction in cancer-related mortality between 1991 and 2017 [3]. Current standard treatment plan for metastatic cancer includes usage of pharmaceutical chemotherapeutic agents, surgical resection, radiation and targeted therapy on growth factors [7]. The remedies, especially pharmaceutical chemotherapeutic drugs are often more expensive than natural alternatives, limiting their accessibility, especially in resource-constrained settings [2]. Cancer patients from low- and middle-income countries often resort to risky financial decisions due to the burden of high medication costs, repeated visits, and palliative care [8,9]. Despite advances in cancer treatment, current strategies frequently cause more harm than good, with high recurrence rates persisting even after surgery and medication [10]. A vital component of anti-cancer treatment is chemotherapy [11]. Nevertheless, a critical issue known as “drug resistance” has emerged in the realm of cancer chemotherapy [12]. Frequent exposure to chemotherapeutic agents can render cancer cells unresponsive to their inhibitory effects [12]. To counteract this challenge, high doses of these agents are administered to impede cancer progression. However, the use of elevated doses has a drawback - it leads to dose-dependent side effects, imposing limitations [12]. Most of the second- and third-stage cancer patients often fail to meet the inclusion criteria for clinical studies because they are heavily medicated and at risk for multi-drug resistance [13]. Multiple anti-cancer drug resistance accounts for approximately 9 in every 10 cancer-related deaths [13]. Also, there is higher clinical complexity for cancer patients compared to non-cancer patients as the patients often require frequent admissions to acute care hospitals [14]. The length of stay and readmission rates are higher for cancer patients compared to non-cancer patients, making them highly exposed to the pathogen infection, causing bloodstream infections, often ending with sepsis [14,15]. Infections are mostly caused by Gram-negative bacteria [15], thus generating more interest in developing cancer theranostics with dual properties that can work for both bacterial infections as well as for the cancer, as seen in case of lung cancer patients being administered boron and zinc derived nanomaterials for their anti-bacterial properties [16]. Nanotechnology has been increasingly viewed as a game-changer, with as many as around 75 anti-cancer formulations in clinical trials, with approximately more than 15 of them FDA approved [17]. However, there is still ongoing research as there are many nanoformulations that have not received approval for clinical application due to their toxicity in tissues and difficulties in obtaining the ideal nano-formulation size for optimum anti-cancer activity; with additional properties such as anti-bacterial activities also being needed in combinatorial therapies for different types of cancer [17].
The war on cancer is far from being won, and the evolution of cancer theranostics depends on our ability to innovate, drawing inspiration from both modern science and nature [13]. Cancer theranostics combines diagnosis and therapy to enhance personalized cancer care by streamlining treatment, reducing durations, and improving outcomes through early intervention during diagnosis [18]. The purpose of this review is to bring together nanomaterials that can be used for sustainable cancer theranostics. Protein based nanomaterials are majorly known for their non-toxicity and green synthesized nanoparticles are known to be highly efficient in low dosages. Together they can be considered for safe and environmentally friendly cancer treatment that can potentially add more years to the cancer prognosis. The new age of cancer theranostics would benefit from harnessing the synergestic effect of the hybrid nanosystems comprising of proteins and green synthesized nanoparticles. The principles behind their synergy are chemical bonding, self-assembly, in-situ incorporation and adsorption [19-21]. Their synergestic effect offers better performance in properties [22] such as tumor imaging, diagnosis, drug delivery and controlled drug release; all of which is necessary for a biocompatible cancer theranostics [23]. Additionally, the side-effects due to microbial infection can be combated with the combinatorial approach of these nanomaterials. This review describes each nanomaterial's potential and application, overall limitations and possible combinatorial approaches, that can bring together possible candidates to develop sustainable cancer theranostics as appropriate.
Harnessing synergistic nanoparticles from nature
Naturally derived remedies have stood the test of time, with approximately 85% of the global population relying on them [24]. Their affordability, minimal side effects, and ease of preparation make them highly valuable across diverse geographic and socioeconomic communities [24]. Furthermore, these remedies pose no significant risks to human health or the environment [25].
In recent years, there has been a growing preference for naturally synthesized nanomaterials, primarily due to their cost-effectiveness, safety, and low systemic toxicity [17]. Nature offers a vast arsenal of proteins and extracts from plants and animals, which can be harnessed to create anti-cancer nanoscale molecules with high functional synergy. The scientific community has shown increasing interest in hybrid nanostructures combining proteins and nanoparticles [26]. Four key properties: shape, size, charge, and surface functionalization—collectively determine how a protein will “experience” or interact with a given nanoparticle, significantly influencing protein adsorption, conformational changes, and biological responses [26]. Preliminary studies suggest that when nanoparticles come into contact with proteins, a corona layer forms around them, enhancing their stability [26]. Strong interactions are observed in colloidally stable nanosystems, where proteins contribute to nanoparticle stabilization [26]. Recent research has highlighted the role of hydrophobic forces, hydrogen bonding, and van der Waals forces in facilitating these interactions [27]. Human serum albumin has demonstrated remarkable stability as a coating protein, with hybrid nanoparticles maintaining stability in water, Dulbecco's phosphate-buffered saline, and Dulbecco's modified Eagle medium [26]. Several studies have supported the viability of hybrid nanosystems involving human serum albumin and metals such as silver, copper, iron, zinc, and even rare metals like niobium [26-30]. The use of protein nanostructures as carriers for green nanoparticles presents promising opportunities for sustainable and safe cancer theranostics. Plant molecular farming enables plants to produce proteins, expanding the scope for combinatorial approaches [31]. Under optimal conditions, metal nanoparticles and protein nanostructures can self-assemble to form hybrid multimeric nanomaterials [32]. In these hybrids, proteins often serve as both linking and reducing agents for the metal nanoparticles [33]. This synthesis is achievable through protein-nanoparticle co-engineering, leveraging the self-assembly principle of biological systems [34,35]. Self-assembly involves intermolecular interactions such as van der waals forces [36], Hydrogen bonding [37], Ion-dipole interactions [38,39], Ion-Induced dipole interactions [40], and Dipole-induced dipole interactions [19]. Inorganic materials can be combined into organic materials while synthesizing, through in-situ incorporation [20]. The materials can also be adsorped, which makes the amalgamation of the nanomaterials easier [20].
Although the available literature is still in its early stages, more in vitro and in vivo studies are needed to fully explore the biomedical applications of these hybrid nanosystems. Complementary research investigating their additional properties will serve as a foundation for developing novel hybrid nanostructures. For instance, a study by Rezazadeh et al. (2020) demonstrated enhanced functional properties of silver nanoparticles when combined with chitosan-algae extract through biosynthesis. The synergy between the polysaccharide-based extract and nanoparticles not only controlled the size of the silver nanoparticles but also increased their bioavailability [41]. Furthermore, for effective antimicrobial activity, nanomaterials must either directly interact with microbes or facilitate the entry of antimicrobial agents [42]. Characterization studies of green-synthesized nanoparticles have shown promising antimicrobial properties, with high stability even without capping agents [43]. Notably, silver nanoparticles combined with bacteriophages (which consist of nucleic acid enclosed in a protein coat) have demonstrated efficacy in preventing secondary infections caused by Salmonella, a Gram-negative bacterium [44]. Gliadin based silver nanoparticles have been assessed for their anti-bacterial properties, which can be also extended to anti-cancer studies [45].
Studies have also shown that plant-derived silver nanoparticles exhibit superior antimicrobial activity compared to plant extracts alone [46]. Additionally, silver nanoparticles conjugated with albumin, collagen, zein, and lysozyme have demonstrated remarkable drug cargo uptake by osteosarcoma cell lines [47]. Small molecules, proteins, peptides, and nucleic acid sequences can be modified through mosaic, vertex, capsule, or cantilever approaches to function as effective carriers for diverse anti-cancer nanoparticles, including metals [48]. Furthermore, silver nanoparticles conjugated with Hepatitis B core antigen protein via an in vivo asymmetric self-assembly strategy could be used to develop immunoassays for diseases, including cancer [49]. Zinc-Chitosan nanoparticles exhibited stronger antibacterial effects, with minimal inhibitory concentrations of 9.25-13.5 µg/mL, completely inhibiting Staphylococcus aureus and Escherichia coli [50]. In anticancer activity, Zinc-Chitosan nanoparticles triggered apoptosis in human acute T-lymphocyte leukemia cells, leading to 65-70% cellular damage [50]. The results suggest that Zn-CSNPs hold promise as a therapeutic approach for treating zinc-deficiency-related diseases, particularly human acute leukemia [50].
Early studies have also demonstrated the cytotoxic potential of gold nanoparticles stabilized by gelatin for delivering adriamycin to leukemia cells [51]. Drug internalization has been confirmed in gold-silica nanoparticles conjugated with human serum albumin, loaded with Doxorubicin, and sealed with Rose Bengal, creating a multifunctional nanosystem for diagnostics and therapeutics [52]. An in vivo study further validated the potential of gold-human serum albumin conjugates as a controlled drug delivery system for metastatic colorectal cancer [53].
These findings collectively highlight the immense potential of hybrid nanosystems in biomedical applications, particularly in cancer therapy. However, further research is essential to refine their design and ensure their safety and efficacy in clinical applications.
Protein based nanostructures
There are many ways we can describe the existence of living beings. In one such narrative, proteins are the building blocks of all living beings [54]. Protein structures can vary from simple monomeric proteins to more complex arrangements like oligomers, polymers, and networks formed through interactions between proteins [55,56] (Figure 1). It is not a surprise that proteins can be used in a beneficial way, such as, a part of cancer treatment process, in form of protein nanostructures [57].
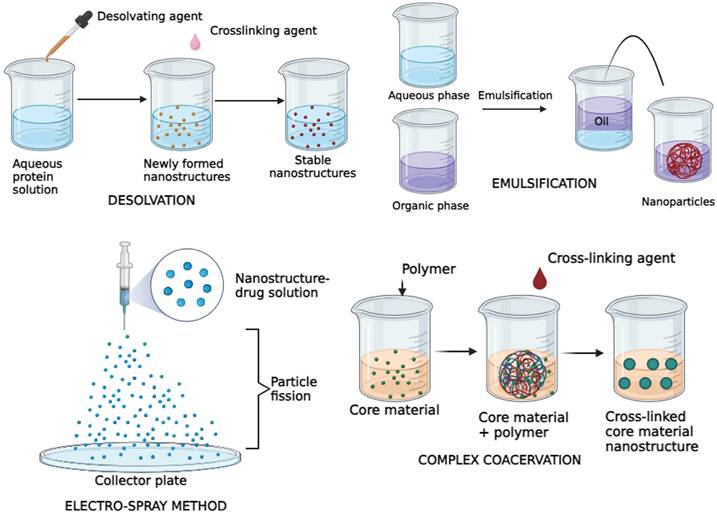
Proteins are abundant in nature, which makes it easier for procurement and synthesis [58,59]. A lot of these protein nanostructures are made using substances such as Albumin, Collagen, Gelatin, Legumin, Elastin, Ferritin, Soybean, Milk protein, Zein and Gliadin [60,61]. Although the raw materials involved are proteins, they are reinforced to be structurally stable and customized to function, giving the desirable effect [58,59]. The protein nanostructures are synthesized using techniques such as desolvation, emulsification, electrospray method and complex coacervation [62,61] (Figure 2). The choice of preparation methods, site-specific modifications, and recombinant engineering for these nanostructures is influenced by the physicochemical properties and composition of the therapeutic agents and proteins [61].
Both naturally occurring and synthetic biomolecules, such as polysaccharides, nucleic acids, peptides, and proteins, have been investigated for the purpose of creating nanostructures in the field of cancer nanomedicine [63,64]. Tetrahedral DNA nanostructures (TDNs) have gained global attention for their stability, biocompatibility, and ease of modification, making them versatile carriers for various therapeutic agents and imaging probes in drug delivery, molecular diagnostics, and biological imaging [65].
Nanostructures formed from simple to complex proteins.
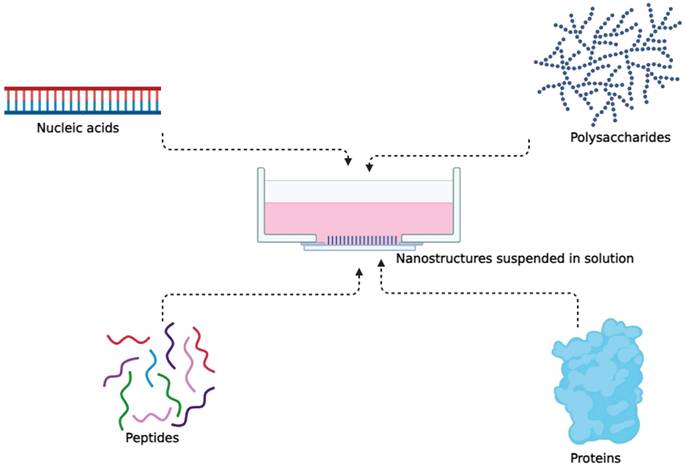
Illustration depicting major types of protein nanostructure synthesis methods.
Nature has evolved a diverse repertoire of protein nanostructures over millions of years, equipping them with a multitude of functions [55]. For example, viral capsids are a striking example of this phenomenon, as they are constructed from multiple copies of a monomeric protein unit, forming sturdy polyhedral structures crucial for safeguarding and transporting genetic material [66]. Similarly, bacterial exotoxins like botulinum toxins consist of distinct structural domains, each serving a specific function, collectively making them potent weapons in the natural world [67]. What adds to the intrigue is that post-translational modifications can further enhance the diversity of protein building blocks, expanding the array of functional nanostructures [57].
The development of well-defined nanostructures with bioactivity and favorable material properties is particularly significant in biomedicine, where stability, biocompatibility, biodegradability, functionality and biosafety are of paramount importance [68-70].
Protein nanostructures in cancer therapeutics
Protein nanostructures are catalysts for multiple complex cellular mechanisms, through their function, molecular recognition and stable structural frameworks [69]. Stability is very important, as they should never degrade in the host environment, which in this context would be the body of the cancer patient [71]. Whether it is to deliver drugs to the site of tumor or to induce a cellular process, structural stability is there in protein nanostructure [72,73].
At lower doses, protein nanostructures perform well and there are usually lesser chemical reactions [62]. Protein nanostructures also provide a solution to alleviate the side effects associated with anti-cancer therapy such as chemotherapy [74]. They achieve this by selectively homing in on tumor cells, leaving healthy cells unharmed, thereby enhancing therapeutic effectiveness [75]. Human serum albumin, one of the most common protein nanostructures used to deliver nanoparticles, is reported to enhance the anti-cancer and anti-bacterial properties, with least toxicity [76].
Protein nanostructures offer significant advantages in biomedical applications due to their remarkable multifunctionality. Their adaptable design allows for the integration of various functionalities within a single platform, making them particularly valuable for addressing complex diseases like cancer [77]. These functionalities include targeted drug delivery, sequential targeting, responsiveness to stimuli, theranostics (combining therapy and diagnostics), combination treatments, and the incorporation of logic gates for precise therapeutic actions [61].
Human serum albumin
Human Serum albumin, the most abundant protein in the blood, is a potential candidate for medication administration. It has a half-life of 19 days [78]. Through GP60-receptor-mediated transcytosis, it specifically binds to the secreted protein acidic and rich in cysteine, enabling it to efficiently enter vascular endothelial cells and selectively accumulate inside cancer tissues [79]. According to Wang et al. (2021), via in vivo investigations, it has been shown that a nanovaccine composed of endogenous human serum albumin and specific constituents enhances both innate and adaptive immunity against several types of cancer. Albumin nanocarriers offer numerous advantages, including non-toxicity, biodegradability, ease of preparation, non-immunogenicity, precise sizing, and the presence of reactive groups like thiols, amines, and carboxyl groups [80]. Various albumin-based cancer nanomedicines derived from anticancer formulations have been implemented in clinical trials, demonstrating the promise of albumin in treating cancer. The FDA has approved Abraxane, a nanomedicine that uses albumin as its basis, for the treatment of advanced breast, lung, and pancreatic cancer [61].
Saleh et al. utilized the desolvation method to craft human serum albumin (HSA) nanoparticles containing curcumin, which were then delivered to HER-2 positive breast cancer cells [80]. The drug-loading efficiency was 3.4%, with an encapsulation efficiency of 71.3%, leading to enhanced stability and solubility of curcumin. Surface modification enabled targeted delivery by conjugating HER2 Apt to the nanoparticle surface [80]. Ex vivo experiments confirmed that these albumin-loaded nanocarriers improve drug release, increased bioavailability, enhanced pharmacokinetic properties, and improved drug targeting to tissues [80]. The use of HSA nanoparticles as vectors to transfer genes or antibodies has also been investigated. In a study, Mesken et al. used HSA nanoparticles coupled with a cell-penetrating peptide (CPP) to transfect HEK 293T cells. These nanoparticles were also generated using the desolvation process, with particle sizes ranging from 207.8 ± 21.3 to 222.8 ± 42.4 nm [81]. Plasmid loading efficiency was confirmed in vitro to be unaffected by CPP surface alteration, with a value of 78.3 ± 13.0% [81]. At high plasmid concentrations, transfection efficiency rose by up to 50% [81]. Furthermore, HSA nanoparticles can be used as carriers of non-DNA payloads due to their low cytotoxicity. For instance, Redín et al. created HSA nanoparticles that were loaded with the chemical medication bevacizumab, which is used to treat certain eye illnesses and tumours [82]. The resultant bevacizumab showed high stability and a two-phase release pattern, with a slower, continuous release occurring for more than 24 hours after an initial release of roughly 400 μg/mL during the first 5 minutes. Crucially, in vivo investigations verified the albumin nanoparticle's non-toxicity and showed mucosal adherence [62]. It is important to note that albumin nanoparticles and other nanostructures produced from natural proteins have been used in medicinal applications in the past due to their inherent properties such as drug encapsulation, targeted delivery, stimulus-responsive conformational changes, synergistic theranostics, and enzymatic performances [83].
Gliadin
Gliadin is a gluten protein produced from wheat, showing promise as a polymer for oral and topical drug delivery systems [84]. It is often used in mucoadhesive formulations because of its ability to adhere to mucous membranes. Gliadin offers several appealing attributes, including biocompatibility, biodegradability, natural origin, non-toxicity, and stability, making it an excellent candidate for drug delivery systems [84].
There have been few studies that conducted studies involving gliadin nanostructures loaded with anticancer drugs, performing in vivo experiments that induced apoptosis in breast cells [85], with combination of gelatin [86]. Using an electrospray deposition method, their study was centred on creating gliadin and gliadin-gelatin composite nanostructures for the regulated release of the anticancer medication cyclophosphamide. Gliadin nanostructures containing cyclo-phosphamide exhibited a 48-hours release pattern, while gliadin-gelatin nanostructures exhibited faster release kinetics [85]. Breast cancer cell cultures treated with cyclo-phosphamide-loaded gliadin nanostructures were maintained for 24 hours, resulting in cell apoptosis [85].
DNA
Because of its unique benefits and remarkable biocompatibility, co assembling proteins and DNA to form hybrid nanostructures is a rapidly expanding topic of study. When it comes to creating a variety of nanostructures, DNA is more precise than other nanomaterials. Conversely, proteins offer a multitude of well-established specific biological functions [87]. Consequently, DNA-protein nanostructures enjoy the unique advantage of utilizing DNA as a structural scaffold for the precise generation of predicted nanostructures, while accurately labeling proteins to perform various functions [87]. This synergy results in the development of novel hybrid nanomaterials with functionalities that cannot be achieved by individual biomolecules [87].
One noteworthy instance is the self-assembly of hybrid nanospheres made of DNA and streptavidin, which are highly versatile and can easily load chemotherapeutic drugs and functionalize with targeting molecules. In a one-pot self-assembly system, the nano-spheres go through three reaction phases [88]. Doxorubicin (Dox), a popular cytotoxic chemotherapy drug, may be loaded into these nanospheres with ease [88]. The loaded nanospheres, also called Dox-nanospheres, show traits of continuous drug release [88]. Because of their exact modularity for in vivo imaging and cancer targeting, their biocompatibility, and their easy one-pot synthesis, self-assembled DNA-streptavidin hybrid nanospheres offer tremendous potential as a cancer-targeted nanoplatform [89].
Peptide based hydrogels
Peptide hydrogels have emerged as frontrunners in the realm of medical applications, owing to their remarkable structural and functional attributes. Numerous self-assembling peptides have been developed, holding promise as carriers for delivering anticancer drugs [90]. These self-assembled peptides form hydrogels with nanotube-like structures. Extensive examinations have confirmed their mechanical robustness, stability, biocompatibility, and precise microscale dimensions. Additionally, these nanotubes exhibit thermal and chemical properties well-suited for their intended purpose [90].
An important milestone was reached by Mao et al., who pioneered a drug delivery system centered on a self-assembled peptide hydrogel. Their research successfully integrated two chemotherapeutic drugs, resulting in a notable enhancement of drug safety [91]. This innovative device achieved controlled drug release through ester bond hydrolysis, showcasing its potential for precise and targeted anticancer drug delivery. Small peptide hydrogels are regarded as more advantageous for drug delivery due to their cost-effectiveness and customizable properties [92].
Collagen and gelatin
Collagen nanoparticles (collagen-NPs) are gaining recognition as promising biopolymeric nanoparticles due to their biodegradability and biocompatibility, characterized by low immunogenicity and non-toxicity. In a study, researchers isolated eight dis-tinct actinomycete strains from soil samples in Egypt, five of which demonstrated the ability to synthesize collagen-NPs [93]. Among these, one strain, identified as Streptomyces xinghaiensis NEAA-1, exhibited the greatest biosynthetic potential, displaying anti-hemolytic, antioxidant, and cytotoxic properties for HCT116 cell lines. In vivo experiments indicated that collagen-NPs could suppress the growth of Ehrlich ascites carcinoma in mice, and when combined with doxorubicin (Dox), they achieved substantial tumor suppression [93].
Moving to another aspect, gelatin (degrade form of collagen) nanostructures loaded with paclitaxel offer controlled drug solubility both in vitro and in vivo settings, especially in aqueous environments, with a sustained release pattern [94]. When administered intravesically, these paclitaxel-loaded nanostructures efficiently target bladder tumors while minimizing systemic absorption [94]. Furthermore, these nanostructures maintain a consistent release of paclitaxel, addressing concerns related to drug dilution. This sustained release has the potential to reduce treatment frequency due to the prolonged maintenance of therapeutic drug levels [94].
Bacteria
Acoustic protein nanostructures, or gas vesicles (GVs), are aerated protein shells found in aquatic bacteria [95]. These nanoscale structures enable strong ultrasound contrast, deep tissue penetration, and multimodal imaging. Genetically encoded GVs offer high-resolution, background-free imaging and can be customized for targeted applications [95]. A group of researchers modified E. coli to gas vesicles (GVs) that target the tumor's hypoxic environment, enhancing ultrasound imaging and enabling focused ultra-sound ablation. Nanoparticles with IR780, perfluorohexane, and AQ4N exploited the hypoxic environment to boost therapeutic effects [96-98]. GVs-E. coli accumulates in tumor regions, improving treatment precision while reducing side effects. The approach was validated through fluorescence, photoacoustic, and ultrasound imaging, demonstrating its diagnostic and therapeutic potential [99]. Similarly, gas vesicles and functionalized GVs produced by cyanobacteria are used in sonodynamic therapy for cancer, enhancing ROS production and tumor growth inhibition [100]. GVs' hollow structure improves ultra-sound contrast, functionalizes with dyes, and assists in imaging and therapy. In vitro and in vivo studies show that GVs effectively induce apoptosis and inhibit tumor growth while maintaining biocompatibility [100].
Soy protein
A study on soy protein isolate (SPI)-based nanoparticles used a pH-driven method to enhance curcumin's stability and bioavailability [101]. SPI-Cur nanoparticles exhibited strong anticancer activity against HepG2 cells by triggering ROS-induced, mitochondria-mediated caspase apoptosis [101]. biocompatibility [100].
Other protein nanostructures
Researchers explored stability, circulation, and tissue distribution of chemically self-assembled nanorings (CSAN) made from dihydrofolate reductase fusion proteins for potential in vivo applications [102]. In vivo microPET/CT imaging revealed significant tumor accumulation and high-contrast imaging, demonstrating CSANs' potential for drug delivery and imaging [102]. Protein based nanostructures enhance immune responses by targeting antigen-presenting cells and delivering therapeutic agents [57]. Dual-targeting nanoparticles with monoclonal antibodies offer a stable, cost-effective alternative to bispecific antibodies, enabling more precise cancer treatment [103]. Virus-like nanoparticles also show promise for antigen delivery in cancer immunotherapy [104]. One study examined bacteriophage protein-based nanotubes as therapeutic nanocarriers [105]. While colorectal cancer cells internalized them, primary macrophages cleared them, posing a challenge for therapy. Reduced macrophage clearance with age suggests potential for elderly cancer patients [105]. Ferritin, a well-studied protein with an octahedral structure, can encapsulate drugs and target tumor cells via transferrin receptors [106].
Limitations of protein nanostructures
However, using nanostructures in cancer therapy comes with challenges. Biological barriers can impede their efficient transport to target tissues, reducing delivery efficiency. Nanoformulations are also vulnerable to clearance by the reticuloendothelial system and often struggle with limited penetration into tumors compared to free drugs [107]. Researchers have focused on developing functionalities to overcome these barriers and enhance tissue penetration [107]. A multitude of factors may increase the risk of an unexpected loss of function or adverse effects, and the diverse range of nanomaterials may lead to intricate in vivo behaviours; therefore, careful consideration is necessary when developing multifunctional nanomaterials, considering the specific medical requirements as well as the unique properties of the materials [107]. Another major limitation while using protein nanostructures is the difficulty adjusting its size and the high energies that it has due to its size [62]. Drug delivery vehicles based on natural proteins have several benefits for treating cancer, but clinical translation is still a difficult task. Critical to therapeutic applications include concerns of large-scale synthesis, stability of formulations, in vivo distribution, metabolism, and excretion, as well as structural heterogeneity. To further understand nanostructure absorption and dispersion, sensitive in vivo detection methods are needed [61]. In protein complexes, maintaining activity under mild conditions is crucial [108]. Non-covalent or dynamic covalent strategies preserve structure and function [109,69] enabling reversible assembly to be like in nature [110]. Chemical cross-linking, however, can limit protein dynamics due to stability constraints [111].
According to one study, (Wang et al., 2021) protein engineering suited to particular therapeutic applications and de novo design can overcome constraints associated with the functionalization of nanostructures based on the structures and properties of original proteins [61]. Biotechnology enables tailored protein nanostructures, using genetic engineering and de novo design [112]. Challenges remain in predictability, function integration, and avoiding aggregation [112].
Green nanoparticles
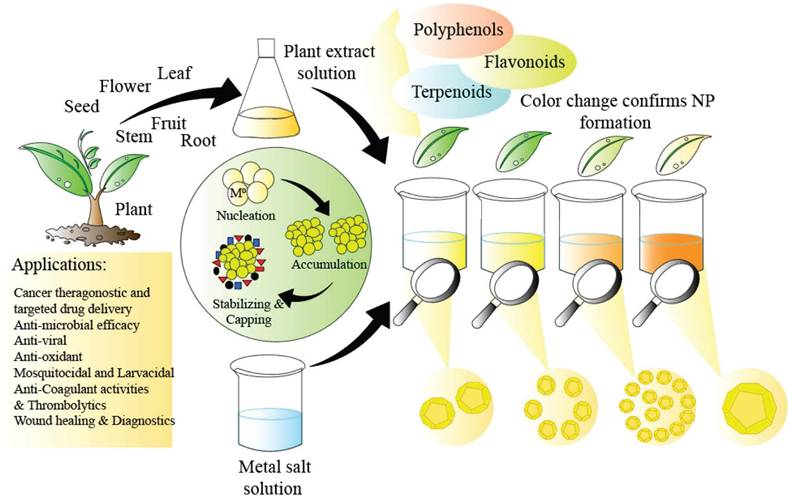
For applications in biology and medicine where nanoparticle purity is paramount, the utilisation of natural resources to produce nanoparticles offers a sustainable, environmentally friendly, cost-effective, and chemical contaminant-free option [113]. It is easy to mass-produce common and useful nanomaterials [113]. There is no need for harmful chemicals in biological procedures. Plant extracts have less harmful byproducts that are easier to get rid of [113]. Moreover, the green synthesis aims for better yields compared to the traditional chemical methods [114]. The yields are mostly metal nanoparticles, and they are preferred for their unique optical, electronic, and catalytic properties [115]. Green synthesis comes under the bottom-to-top approach synthesis process that uses chemical or biological methods, as compared to the top-to-bottom approach that uses physical methods [114]. Nanoparticles can be fabricated biologically using plant extracts, which act as natural reducing agents [116]. The process involves mixing dried, crushed plants with a solvent to obtain the extract, which is then combined with a metal ion solution [114]. This green synthesis method eliminates the need for additional reducing agents as the plant extract facilitates the reduction of metal ions to nanoparticles [116,117] (Figure 3).
Favourable high yield requires the optimal temperature and pH, as temperature controls nucleation while pH controls the growth kinetics and stability [118,114]. Nucleation is the first stage of nanoparticle formation, which determines the size and morphology of the product [114]. Other conditions that also determine high yield are concentration of the reaction, reaction time and the plant extract components [114].
The advantages of using plant extracts are high as they contain natural polyphenols [119], carbohydrates, amino acids and proteins [120], which help in making stable nanoparticles with faster synthesis rate than nanoparticles not using plant extracts [121]. Moreover, plant extracts serve as both reducing and stabilizing and capping agents, reducing aqueous solutions of metal salts into nanoparticles while preventing their aggregation over time [122,123]. Green synthesized nanoparticles, such as those derived from curcumin, are also known to show dual properties of anti-cancer and anti-bacterial activity, in conjuction with silica-coated Fe3O4 magnetic nanoparticles [124].
Green nanoparticles in cancer therapeutics
There is a growing trend of green synthesis that focuses on creating nanoparticles with more stability [69]. Comparing Nanoparticles made using green route synthesis to those made using physico-chemical techniques reveals that the former are more stable and effective [113]. In the spirit of going green, be it with energy, or even with nanotechnology, researchers have found newer methods to design sustainable nanoparticles from renewable sources. There are different categories of nanoparticles that are green in synthesis, with metals from gold, silver, copper and so on (Figure 4).
Gold nanoparticles
Gold nanoparticles (AuNPs) have garnered increasing interest for their potential in bio-medical applications and the focus on green synthesis methods has intensified due to their associated biocompatibility and scalability. Previous studies have proved the anti-microbial activity of gold nanoparticles to increase with higher volume [125]. A study by Ashikbayeva, et.al, describes the synthesis of AuNPs derived from green tea leaves and their subsequent application in the detection of the CD44 cancer biomarker via a biosensor constructed with ball resonator optical fibers [126]. Characterized by its rapid and label-free operation, the biosensor can be fabricated in just 20 seconds and features a compact de-sign that holds promise for in vivo applications [126]. In a study where gold nanoparticles (AuNPs) derived from the leaf extract of Coleus scutellarioides (L.) Benth was considered for breast cancer; the nanoparticles demonstrate effective free radical scavenging capabilities, particularly at higher concentrations compared to the plant extract alone [127]. Furthermore, the cytotoxic effects of the AuNPs were evaluated against the MDA-MB-231 breast cancer cell line, revealing a dose-dependent reduction in cell viability for the cancer cells, while showing no significant cytotoxicity towards normal cells [127]. Morphological changes in cancer cells, such as shrinkage and detachment, were observed after treatment, suggesting selective toxicity [127]. In a study where gold nanoparticles (AuNPs) were synthesized using methanol extracts from Moringa oleifera seeds, there was an emphasis on an eco-friendly one-pot process [128]. The antioxidant activity of the AuNPs was assessed using the DPPH radical stabilization method, and the nanoparticles exhibited a dose-dependent effect on A549 lung cancer cell proliferation showing anticancer property [128].
Silver nanoparticles
One such innovation is the green-synthesized silver nanoparticles (AgNPs) from Cucurbita spp. fruit peels [129], providing an environmentally friendly and cost-effective method. An experimentally synthesized Ag-NPs demonstrated a spherical morphology and stability, confirming their biocompatibility to be used as radiosensitizer for triple negative breast cancer [129]. These nanoparticles led to increased expression of apoptotic-related genes in MDA-MB-231 cells and activated the apoptosis pathway and induced endoplasmic reticulum stress, which contributed to enhanced radiosensitization [129]. Another study focused on the anticancer effects of silver nanoparticles synthesized from Andrographis macrobotrys, specifically targeting A549 lung cancer cells [130]. The results demonstrated a pronounced dose-dependent increase in cytotoxic effects, with morphological changes induced in the treated cancer cells, revealing signs of cell shrinkage, membrane blebbing, and apoptotic surface formation [130]. Another study evaluated the anticancer effects of silver nanoparticles derived from lemon balm leaves, graphene, and silver-graphene nanocomposites on MCF-7 breast cancer cells using the MTT assay to measure cell survival [131]. Silver nanoparticles demonstrated significant cytotoxicity, notably increasing cancer cell death correlating with elevated reactive oxygen species and malondialdehyde levels, alongside decreased glutathione [131]. These mechanisms promoted apoptosis in breast cancer cells [131]. In another landmark study, the antioxidant potential of AgNPs synthesized from Moringa peregrina leaf extract, showed strong cytotoxicity activity against MCF-7 breast cancer cells [132]. The AgNPs induced cell death primarily through apoptosis rather than necrosis, influenced by oxidative stress from reactive oxygen species and modulation of key signaling pathways like the p53 gene [132]. Additionally, AgNPs was found to impede tumor migration and angiogenesis, potentially reducing the risk of metastasis in cancer treatments [132]. A group of researchers synthesized silver nanoparticles from red seaweed Champia parvula extract, leveraging its antioxidant-rich phytochemicals like phenols, flavonoids, and tannins [133]. These Champia parvula-mediated AgNPs (Cp-AgNPs) showed stability and antimicrobial activity against Streptococcus mutans, Staphylococcus aureus, and Candida albicans, and anticancer effects, especially against lung cancer cells indicating potential as multifunctional cargo [133].
Copper nanoparticles
Synthesis of copper oxide nanoparticles for cancer therapy is also an underexplored area, and recently, significant advancements have been made in utilizing copper oxide nanoparticles (CuONPs) for gene delivery applications [134]. Looking at a study that confirmed the stability of conjugated copper oxide nanoparticles (CuONPs) with folate, there was significant cytotoxicity observed against MDA-MB-231 breast adenocarcinoma cells by induced apoptosis and reactive oxygen species. The nanoparticles were synthesized using Staphylococcus aureus extracts [135].
There are studies where copper nanoparticles were characterized for anti-microbial activity [136] and were tested against seven strains of microbes, with the right sized nanoparticles, and fast synthesis [137]. In a recent study, CuONPs were biologically synthesized using leaf extract from Melia azedarach, followed by functionalization with chitosan and polyethylene glycol [134]. The CuONPs were then conjugated with folate as a targeting ligand to enhance their specificity for cancer cells [134]. In vitro studies assessing cytotoxicity showed that the CuONPs maintained cell viability greater than 70% across various cell lines, including human embryonic kidney (HEK293), breast adenocarcinoma (MCF-7), and cervical cancer (HeLa) cells [134].
Another study explored the phytochemical-assisted synthesis of copper nanoparticles (CuNPs) using the stem extract of Hippophae rhamnoides, a plant indigenous to the Himalayas and known for its rich phytochemical profile [138]. Further investigation into the CuNPs' anticancer potential was conducted on HeLa cell lines [138]. Results from the MTT assay indicated a dose-dependent cytotoxic effect with an IC50 value of 48 µg/mL, underscoring a strong inhibitory impact on cancer cells [138].
Thematic illustration of how green synthesized nanoparticles are formed [117].
Different types of green synthesized nanoparticles, with their biological extract origin and metal ions showed.
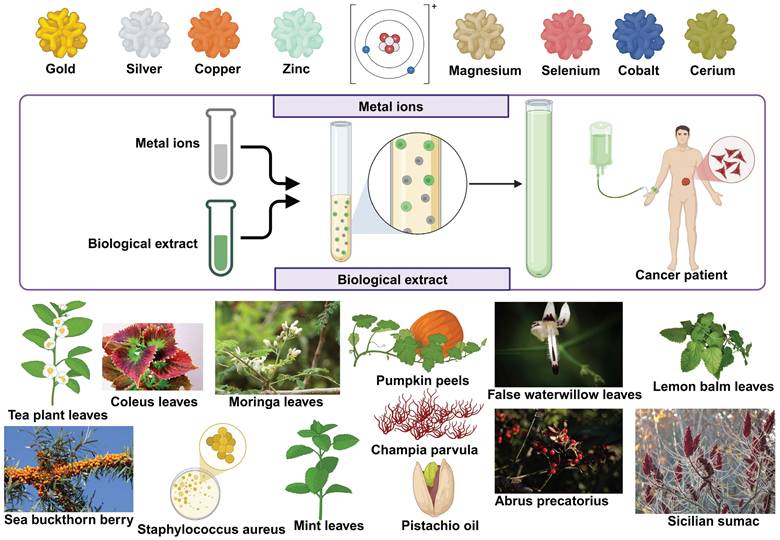
Zinc nanoparticles
Cytotoxicity assays conducted on HT-29 colon cancer cells revealed that ZnO(Zinc Oxide) nanoparticles synthesized with Artocarpus hirsutus seed extract exhibited potent dose-dependent cytotoxic effects [139]. The nanoparticles triggered reactive oxygen species generation, leading to oxidative stress that damaged cellular structures and pathways critical for cell survival [139]. This oxidative environment contributed to the downregulation of the anti-apoptotic Bcl-2 gene, promoting apoptosis in cancer cells [139]. Additionally, the study highlighted morphological changes associated with apoptotic cell death, supporting the mechanism of action [139]. The study by Mongy et. al investigated the biogenic synthesis of zinc oxide nanoparticles (ZnO NPs) using Rhus coriaria fruit extracts, demonstrating their eco-friendly production and potent anti-cancer effects on breast cancer cells, MCF-7 and MDA-MB-231 [140]. Mechanistic studies indicated that ZnO NPs induce apoptosis in MDA-MB-231 cells, as evidenced by significant nuclear fragmentation, increased apoptotic populations, and S-phase arrest [140]. Additionally, the ZnO NPs significantly hindered the colony-forming ability of MDA-MB-231 cells and their wound healing capabilities, indicating promising anti-migratory properties [140]. Zinc nanoparticles also exhibit anti-microbial activity, causing bacterial cell death as soon as they interact, and the process has been experimentally known to be the simplest, in comparision with other nanoparticles [141].
Magnesium nanoparticles
Magnesium oxide nanoparticles (MgO NPs) are garnering increasing attention compared to other metal oxide nanoparticles due to their unique properties and diverse applications [142]. Their enhanced stability-to-weight ratio, lightweight nature, and recyclability make them particularly appealing for various fields, especially in biological applications [142]. MgO NPs are nontoxic and hygroscopic, which further contributes to their utility in bio-medical contexts [142]. In a study, the synthesis of magnesium oxide nanoparticles utilizing the bark extract of Abrus precatorius demonstrated high efficacy of treatment with MgO NPs inducing both apoptosis and necrosis in a concentration-dependent manner [143]. Cytotoxicity investigations revealed the cytotoxic effects of MgO NPs were time- and dose-dependent [143]. Notably, the MgO NPs induced reactive oxygen species formation, leading to DNA damage and subsequent apoptosis in the A375 cell line [143]. Magnesium nanoparticles have additional anti-microbial activitiy against food-borne pathogens [144].
Other metallic nanoparticles
In recent years, spinel ferrite nanoparticles have garnered significant attention for their promising applications in biomedicine, particularly in cancer treatment through plasmonic photothermal therapy [145]. This innovative approach utilizes nanoparticles that exhibit strong absorption in the infrared region, enabling localized heating upon laser irradiation [145]. Among various spinel ferrite nanoparticles, cobalt ferrite (CoFe₂O₄) and zinc ferrite (ZnFe₂O₄) have emerged as notable candidates due to their unique magnetic and thermal properties [145]. The efficacy of these biosynthesized nanoparticles was evaluated against MCF-7 breast cancer cells in conjunction with laser radiation; revealing a significant reduction in cancer cell viability, with CoFe₂O₄ nanoparticles exhibiting greater photothermal efficacy compared to ZnFe₂O₄ [145]. Both nanoparticles showed acceptable biocompatibility with normal cells, emphasizing their potential for safe biological applications [145]. The cytotoxicity mechanism is primarily attributed to the generation of reactive oxygen species, inducing oxidative stress and disrupting cellular functions, while ZnFe₂O₄ nanoparticles demonstrated enhanced efficacy in inducing cell death, likely due to zinc's role in cellular metabolism and tumor suppression pathways [145]. Another promising anti-cancer study investigated the synthesis of cerium oxide nanoparticles (CeO2 NPs) utilizing pistachio Vera Pericarp essential oil as a coating, focusing on human prostate (LNCap) and breast cancer (MCF7) cell lines [146]. The biological assays demonstrated that CeO2 NPs exhibited significant cytotoxic effects on LNCap and MCF7 cells, with a marked decrease in cell viability observed when used in conjunction with zoledronic acid [146]. These nanoparticles affected cell proliferation, apoptosis, and migration by regulating the expression of apoptosis-related genes BCL-2 and BAX, as evidenced by real-time PCR analysis [146]. Specifically, treatment with CeO2 NPs resulted in a reduction of BCL-2 expression and an increase in BAX expression, thereby promoting apoptosis [146]. The results indicate that CeO2 NPs, especially when combined with ZA, could be effective therapeutic agents for treating prostate and breast cancers [146]. The anticancer properties of selenium nanoparticles were evaluated using human breast cancer MCF-7 cell lines [147]. At a concentration of 500 mg/mL, SeNPs significantly reduced cell viability, lowering it to 61.2 ± 2.2% after 24 hours of exposure [147]. This indicated a substantial cytotoxic effect on cancer cells, suggesting that SeNPs could potentially inhibit cancer cell proliferation [147]. The results highlight the potential of SeNPs as a promising agent for cancer treatment, especially for breast cancer, where targeted nanoparticle therapies are of growing interest [147]. Researchers synthesized pure and Cobalt-doped Nickel Oxide nanoparticles (1%,3%, and 5% Co-NiO-NP) using Salvadora persica plant extract [148]. Physicochemical analyses confirmed uniformly spherical nanoparticles at nanoscale. Cytotoxicity tests on MCF-7(breast cancer) and HUVEC (human endothelial) cell lines showed that Co-doped NiO-NP had greater inhibitory effects than pure NiO-NP, with cytotoxicity increasing with cobalt content [148]. These findings support the potential of Co-doped NiO nanoparticles for biological applications, particularly in cancer treatment [148]. A study that examined the effects of Mentha spicata-loaded Fe nanoparticles on LS174t colon cancer cells revealed changes in the expression of pro-apoptotic BAX and anti-apoptotic Bcl2, suggesting a pro-apoptotic impact from the combination of Mentha spicata and Fe nanoparticles [149]. The synthesized nanoparticles demonstrated note-worthy interactions with LS174t cells, showing not only significant cytotoxicity but also alterations in the apoptotic pathway, as indicated by the modulation of BAX and Bcl2 expression [149]. This pro-apoptotic activity, particularly pronounced in the Mentha spicata-loaded Fe nanoparticles, suggests their potential role in enhancing the effectiveness of existing cancer therapies, especially when combined with radiotherapy [149].
Limitations of Green nanoparticles
There is no doubt in establishing the fact that green nanoparticles are sustainable, efficient and have additional properties (Table 1), however, there are certain limitations [150]. There are risks of off-target toxicity as nanoparticles tend to translocate across different barriers with ease [150]. The extent of biochemical reactions caused by the nanoparticles within the human body still needs complete comprehension [150]. Unregulated translocations can result in harmful effects such as oxidative stress, cytotoxicity and genotoxicity [150]. Moreover, sourcing of raw material is sometimes challenging in green synthesis as some natural sources tend to be endemic to particular regions [116]. Long reaction time and consequential energy consumption is also a matter of concern when it comes to the synthesis process [116]. Variable outcomes in terms of particle size, quality and effectiveness are potential issues that can happen during the synthesis process [116]. Since green synthesis can depend on a lot of factors such as pH, temperature and bio-chemical conditions, aggregation is a possibility that can hinder drug efficiency [151]. Proper stabilization and guidance system for green nanoparticles within the body can make a lot of difference in cancer theranostics.
Recent research heralding protein-green nanoparticle nanosystem specific for cancer theranostics
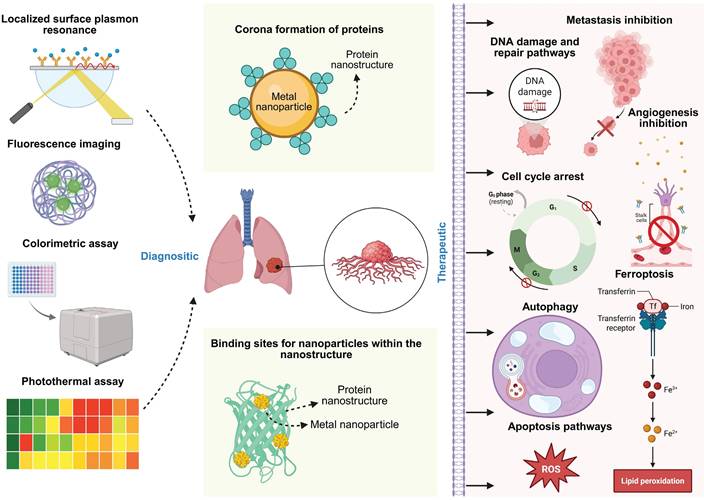
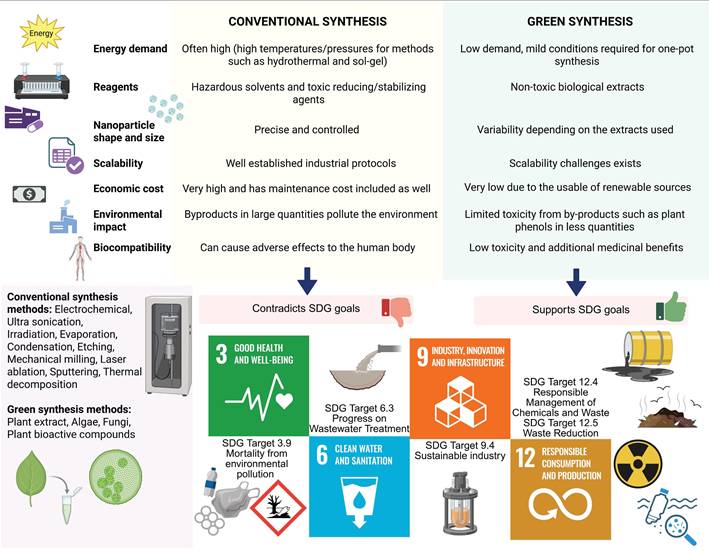
Latest approach involves the use of nanogels made of β-Lactoglobulin to be used as cargo carriers for metal nanoparticles [152,153]. Compared to the pristine nanoparticles, the absorption rate is higher when the metal nanoparticles are conjugated with the nano-gel, expanding the utility of the nanoparticles. Moreover, protein based nanogels can help with easier cell penetration and can be eviscerated by phagocytosis [152,154]. Silver nanoparticles synthesized from clitoria ternatea plant extract combined with sodium alginate and gelatin polymer blends has shown apoptotic activity in lung cancer cell line [155]. Gold nanoparticles derived from Cassia fistula had antimicrobial and anticancer activity against E. coli DH5-Alpha and skin melanoma cell line, while maintaining good stability conjugated with Human serum albumin [156]. Tricholoma crassum derived gold nanoparticles with natural protein coating that show anti-microbial activity against multiple microbes, while selectively binding to sarcoma cells to induce apoptotic activity [157]. The area of protein-green nanoparticle nanosystems is still an area that needs more exploration, particularly conducting in vivo studies and further clinical studies. This biological based method is generally simple and reduces the amount of chemicals used for synthesis. The protein component acts as a stabilizing and reducing agent for the metal nanoparticles, with a focus on targeted drug delivery [158]. Additionally, there is a positive effect of proteins on the synergistic nanoparticle in maintaining the colloidal stability of the nanoparticle [159]. The protein's corona often has additive properties such as accumulation, degradation, inflammation, cellular uptake and clearance, which makes the nano system compatible with complex organism systems like that of human [159]. Nanoparticle specific processes such as glass transition, crystallization, gelation and flocculation which can be influenced by the protein involved [159]. This overall can control the biological reactivity of the nano system, making it safer and sustainable [159]. Design principles state that the nano systems can be used in diagnostics through fluorescence imaging, colorimetric assay, photothermal and localized surface plasmon resonance [160]. They could also cause cell cycle arrest, apoptosis, and cause the tumour to be treated [161] (Figure 5). One of the recent papers talk about green synthesized zinc nanoflowers from Heliotropium indicum extract, coated with albumin being used to induce oxidative stress in melanoma cells [162]. Multifunctionality is possible due to material synergy, the principle behind effective stimuli response for the hybrid nanosystems [163]. In vivo research faces limitations such as large-scale manufacturing, and in vitro studies help in detailed testing in controlled settings [164]. Reproducibility is another challenge due to the involvement of organic compounds [164]. As most of them are usually one pot approaches, they do not create toxic byproducts [165]. However, there are chances of minimal toxicity due to incorrect concentrations, excess metal ions, excess phenols or salts [165]. Nevertheless, the green synthesis methods have an environmental advantage over the conventionally synthesis methods, which is in line with the United Nations's SDGs (Sustainable Development goals) for 2030 [166,167,168] (Figure 6). The United Nations had envisioned 17 goals and 169 targets as part of a blueprint to develop sustainable practices for living [167]. Biosynthesized nano systems are the need of the hour as they sustain major SDG targets such as Good health and well-being (SDG 3), Clean water and sanitation (SDG 6), Industry, Innovation and Infrastructure (SDG 9) and Responsible consumption and production (SDG 12) [167].
Multifunctionality of green nanoparticles derived using green synthesis
| Element | Bioextract origin | Anti-microbial | Anti-oxidant | Anti-proliferative | Anti-inflammatory | Source |
|---|---|---|---|---|---|---|
| Gold | Coleus scutellarioides | - | + | - | - | Al-Mafarjy et al., 2024 |
| Gold | Moringa oleifera | + | + | + | - | Bouttier-Figueroa et al., 2024 |
| Silver | Andrographis macrobotrys Nees | + | + | - | + | Sivakumar et al., 2023 |
| Silver | Melissa officinalis | - | + | + | - | Motafeghi et al., 2023 |
| Silver | Moringa peregrina | - | + | - | - | Al Baloushi et al., 2024 |
| Silver | Champia parvula | + | + | - | - | Viswanathan et al., 2024 |
| Zinc | Artocarpus hirsutus | + | - | - | - | Sampath et al., 2023 |
| Magnesium | Abrus precatorius | + | + | - | - | Ali et al., 2023 |
The overall mechanism of diagnostic and therapeutic nano systems made of protein and metal nanoparticle.
Advantages and disadvantages of green synthesis methods over conventional methods, in line with UN's sustainability goals for 2030. The sustainable development goals that are mentioned in this context include SDG 3: Good health and well-being, SDG 6: Clean water and sanitation, SDG 9: Industry, Innovation and Infrastructure and SDG 12: Responsible consumption and production, all of which support the need of designing biosynthesized nano systems from protein and green synthesis origins [167].
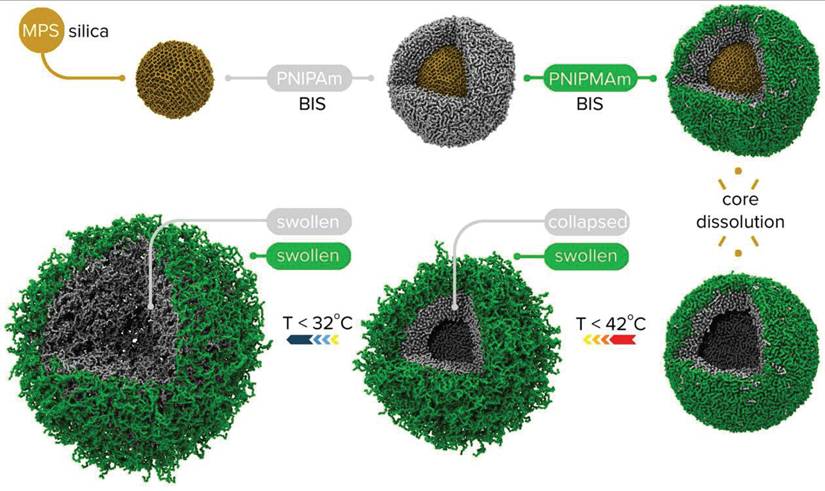
Example of core shell and hollow shell nanogels being synthesized [179].
Future prospects of cancer theranostics: safe and efficient protein-green nanoparticle nanosystems
Nanoparticles derived from green synthesis is an eco-friendly, cost-effective method and utilizes microbes and plants, leveraging their ability to absorb and transform inorganic metal ions [169]. Compared to traditional synthesis methods, green synthesis is more sustainable and biologically safe [169]. A lot of invitro studies are present that strongly establish the anti-microbial and anti-cancer activity of the green synthesized nanoparticles. However, their full potential could be realized through more in vivo studies, especially with their conjugation with protein nanostructures. There are many studies that have proved the anti-cancer and anti-bacterial activities of protein-green nanoparticle nanosystems, where the nanoparticles probably were not green synthesized. These nanosystems come under the class of hybrid nanostructures, which combines different nano materials, enhancing their functionality through unique synergistic properties [170]. They could be core-brush nanoparticles, hybrid nanogels or core-shell nanoparticles, based on the configuration of the nanomaterial produced (Figure 7) [171]. The future research can focus on fine tuning the properties of the nanosystems [172], improve multi-functionality [22], and enhance their native properties [22]. The nanosystems can be designed through sol-gel, solution-phase or through ligand exchange [170]. To make the process easier, artificial intelligence can be used during the research and development process for prediction, modelling, material discovery and design [173]. Using Nano-Quantitative structure-activity relationship (NQSAR) principles, Artificial intelligence and nanotechnology can be brought together for structural characterization as well as toxicity prediction [174]. Artificial neural networks can be used for QSAR, while CORAL can be used for cell viability tests and toxicity prediction [174-177]. Support vector machines can be used for target specification while Logistic linear regression can be used for Adverse outcome pathways [174,177,178]. Nanoinformatics could be leveraged to reduce the time spent in research and development, generating strong candidates for in vivo and pre-clinical studies. The future should focus on establishing a comprehensive pipeline for entirely naturally synthesized nanosystems, minimizing reliance on artificial nanomaterials. This approach would help protect the environment while offering a safer, side-effect-free strategy to alleviate the burden of cancer on humans.
Conclusion
In this review, different types of protein-based nanomaterials and green synthesized nanoparticles are discussed along with their potential in cancer theranostics. The review highlights the promising applications of protein-based nanostructures and green nano-particles in cancer therapy, focusing on their multi-capabilities against cancer and bacterial infections while emphasizing the need for further research to optimize their therapeutic effectiveness. More in vivo studies need to be done as part of future research to create a class of hybrid and efficient nanosystem that is sustainable and safe for humans.
Abbreviations
DNA: Deoxyribonucleic acid; FDA: Food and Drug Administration; GP60: Glyco Protein 60; HAS: Human serum albumin; HER2: Human Epidermal Growth Factor receptor 2; Zn-CSNPs: Zinc-Chitosan nanoparticles; HEK 293T: Human Embryonic Kidney 293 T antigen; MUC1: Mucin 1; HCT116: Human Colorectal carcinoma cell line; microPET/CT imaging: Micro Positron Emission Tomography/Computed Tomography imaging; QSAR: Quantitative structure-activity relationship.
Acknowledgements
We sincerely thank the National Institute of Technology Calicut (NITC) for its continuous support and resources that facilitated this research.
Author contributions
Ashitha Washington: Data curation, Writing-Original draft preparation, Writing -Editing. Gopal Shankar Krishnakumar: Writing -Reviewing. Ravindra Kumar: Conceptualization, Supervision.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA A Cancer J Clinicians. 2024;74:12-49
2. Sharma AN, Dewangan HK, Upadhyay PK. Comprehensive Review on Herbal Medicine: Emphasis on Current Therapy and Role of Phytoconstituents for Cancer Treatment. Chemistry & Biodiversity. 2024;21:e202301468
3. Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clinicians. 2021;71:209-49
4. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-30
5. Bray F, Laversanne M, Sung H. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clinicians. 2024;74:229-63
6. Jokhadze N, Das A, Dizon DS. Global cancer statistics: A healthy population relies on population health. CA A Cancer J Clinicians. 2024;74:224-6
7. Feria A, Times M. Effectiveness of Standard Treatment for Stage 4 Colorectal Cancer: Traditional Management with Surgery, Radiation, and Chemotherapy. Clin Colon Rectal Surg. 2024;37:062-5
8. Wadasadawala T, Mohanty SK, Sen S. et al. Out-of-pocket payment and financial risk protection for breast cancer treatment: a prospective study from India. The Lancet Regional Health - Southeast Asia. 2024;24:100346
9. Del Paggio JC, Naipaul R, Gavura S. et al. Cost and value of cancer medicines in a single-payer public health system in Ontario, Canada: a cross-sectional study. The Lancet Oncology. 2024;25:431-8
10. Mirzayans R, Murray D. What Are the Reasons for Continuing Failures in Cancer Therapy? Are Misleading/Inappropriate Preclinical Assays to Be Blamed? Might Some Modern Therapies Cause More Harm than Benefit? IJMS. 2022;23:13217
11. Anand U, Dey A, Chandel AKS. et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes & Diseases. 2023;10:1367-401
12. Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv Pharm Bull. 2017;7:339-48
13. Tian Y, Wang X, Wu C, Qiao J, Jin H, Li H. A protracted war against cancer drug resistance. Cancer Cell Int. 2024;24:326
14. Numico G, Zanelli C, Ippoliti R. et al. The hospital care of patients with cancer: a retrospective analysis of the characteristics of their hospital stay in comparison with other medical conditions. European Journal of Cancer. 2020;139:99-106
15. Wu J, Song W, Yan H. et al. Metagenomic next-generation sequencing in detecting pathogens in pediatric oncology patients with suspected bloodstream infections. Pediatr Res. 2024;95:843-51
16. Celebi D, Celebi O, Taghizadehghalehjoughi A. et al. Activity of zinc oxide and zinc borate nanoparticles against resistant bacteria in an experimental lung cancer model. DARU J Pharm Sci. 2024;32:197-206
17. Bokhari SS, Ali T, Naeem M, Hussain F, Nasir A. Recent advances in nanoformulation-based delivery for cancer immunotherapy. Nanomedicine. 2024;19:1253-69
18. Al-Thani AN, Jan AG, Abbas M, Geetha M, Sadasivuni KK. Nanoparticles in cancer theragnostic and drug delivery: A comprehensive review. Life Sciences. 2024;352:122899
19. Ananikov VP. Organic-Inorganic Hybrid Nanomaterials. Nanomaterials. 2019;9:1197
20. Palierse E, Masse S, Laurent G. et al. Synthesis of Hybrid Polyphenol/Hydroxyapatite Nanomaterials with Anti-Radical Properties. Nanomaterials. 2022;12:3588
21. Bukhari R. Introduction to Hybrid Nanomaterial: Principle and Application. In: Khanna V, Sharma P, Mahajan P, Eds. Advances in Chemical and Materials Engineering. IGI Global. 2024:183-94
22. Wang X, Jiao X, Wang Y, Feng F, Huang Z, Ge Y. Synthesis and electrocatalytic applications of hybrid nanomaterials containing low-dimensional metals. J Mater Chem A. 2024;12:17002-20
23. Jiang P, Liang B, Zhang Z. et al. New insights into nanosystems for non-small-cell lung cancer: diagnosis and treatment. RSC Adv. 2023;13:19540-64
24. Puri A, Mohite P, Maitra S. et al. From nature to nanotechnology: The interplay of traditional medicine, green chemistry, and biogenic metallic phytonanoparticles in modern healthcare innovation and sustainability. Biomedicine & Pharmacotherapy. 2024;170:116083
25. Nussbaumer S, Bonnabry P, Veuthey J-L, Fleury-Souverain S. Analysis of anticancer drugs: A review. Talanta. 2011;85:2265-89
26. Kopp M, Kollenda S, Epple M. Nanoparticle-Protein Interactions: Therapeutic Approaches and Supramolecular Chemistry. Acc Chem Res. 2017;50:1383-90
27. Akhuli A, Chakraborty D, Agrawal AK, Sarkar M. Probing the Interaction of Bovine Serum Albumin with Copper Nanoclusters: Realization of Binding Pathway Different from Protein Corona. Langmuir. 2021;37:1823-37
28. Wang G, Lu Y, Hou H, Liu Y. Probing the binding behavior and kinetics of silver nanoparticles with bovine serum albumin. RSC Adv. 2017;7:9393-401
29. Perera WPTD, Dissanayake DMRK, Unagolla JM, De Silva RT, Bathige SDNK, Pahalagedara LR. Albumin grafted coaxial electrosparyed polycaprolactone-zinc oxide nanoparticle for sustained release and activity enhanced antibacterial drug delivery. RSC Adv. 2022;12:1718-27
30. Millan S, Susrisweta B, Sahoo H. Probing the interaction between niobium pentoxide nanoparticles and serum albumin proteins by Spectroscopic approaches. Journal of Biomolecular Structure and Dynamics. 2023;41:15435-45
31. Mohammadinejad R, Shavandi A, Raie DS. et al. Plant molecular farming: production of metallic nanoparticles and therapeutic proteins using green factories. Green Chem. 2019;21:1845-65
32. Elangovan A, Suresh D, Tarim AO, Upendran A, Kannan R. Controlled assembly of gold and albumin nanoparticles to form hybrid multimeric nanomaterials. Polymers for Advanced Techs. 2022;33:566-75
33. Zou L, Qi W, Huang R, Su R, Wang M, He Z. Green Synthesis of a Gold Nanoparticle-Nanocluster Composite Nanostructures Using Trypsin as Linking and Reducing Agents. ACS Sustainable Chem Eng. 2013;1:1398-404
34. Mout R, Yesilbag Tonga G, Wang L-S, Ray M, Roy T, Rotello VM. Programmed Self-Assembly of Hierarchical Nanostructures through Protein-Nanoparticle Coengineering. ACS Nano. 2017;11:3456-62
35. Kretzmann JA, Luther DC, Evans CW. et al. Regulation of Proteins to the Cytosol Using Delivery Systems with Engineered Polymer Architecture. J Am Chem Soc. 2021;143:4758-65
36. Margenau H. Van der waals forces. Rev Mod Phys. 1939;11:1-35
37. Emsley J. Very strong hydrogen bonding. Chem Soc Rev. 1980;9:91
38. Zhao L, Song LX, Xia J, Teng Y, Yang ZK, Wang QS. Contribution of polytetrafluoroethylene to the atmosphere-dependent synthesis of Cu-based nanomaterials through ion-dipole interactions. RSC Adv. 2014;4:52836-44
39. Sippel KH, Quiocho FA. Ion-dipole interactions and their functions in proteins. Protein Science. 2015;24:1040-6
40. Kedia A, Kumar PS. Halide ion induced tuning and self-organization of gold nanostars. RSC Adv. 2014;4:4782-90
41. Rezazadeh NH, Buazar F, Matroodi S. Synergistic effects of combinatorial chitosan and polyphenol biomolecules on enhanced antibacterial activity of biofunctionalized silver nanoparticles. Sci Rep. 2020;10:19615
42. Sayed FA-Z, Eissa NG, Shen Y, Hunstad DA, Wooley KL, Elsabahy M. Morphologic design of nanostructures for enhanced antimicrobial activity. J Nanobiotechnol. 2022;20:536
43. Chowdhury MA, Hossain N, Kchaou M, Nandee R, Ahmed Shuvho MB, Sultana S. Scope of eco-friendly nanoparticles for anti-microbial activity. Current Research in Green and Sustainable Chemistry. 2021;4:100198
44. Abdelsattar AS, Hakim TA, Rezk N. et al. Green Synthesis of Silver Nanoparticles Using Ocimum basilicum L. and Hibiscus sabdariffa L. Extracts and Their Antibacterial Activity in Combination with Phage ZCSE6 and Sensing Properties. J Inorg Organomet Polym. 2022;32:1951-65
45. Huang X, Liu B, Ma J. et al. Development of Gliadin@AgNPs hybrid nanoparticles as building blocks for constructing antimicrobial protein-based porous materials. Chemical Engineering Journal. 2024;482:148924
46. Javan Bakht Dalir S, Djahaniani H, Nabati F, Hekmati M. Characterization and the evaluation of antimicrobial activities of silver nanoparticles biosynthesized from Carya illinoinensis leaf extract. Heliyon. 2020;6:e03624
47. Hameed M, Panicker S, Abdallah SH. et al. Protein-Coated Aryl Modified Gold Nanoparticles for Cellular Uptake Study by Osteosarcoma Cancer Cells. Langmuir. 2020;36:11765-75
48. Sharma A, Vaswani P, Bhatia D. Revolutionizing cancer therapy using tetrahedral DNA nanostructures as intelligent drug delivery systems. Nanoscale Adv. 2024;6:3714-32
49. Chen C, Zhou J, Men D, Zhang X-E. Promoter-regulated in vivo asymmetric self-assembly strategy to synthesize heterogeneous nanoparticles for signal amplification. Nanoscale. 2022;14:16180-4
50. Saravanakumar K, Jeevithan E, Chelliah R. et al. Zinc-chitosan nanoparticles induced apoptosis in human acute T-lymphocyte leukemia through activation of tumor necrosis factor receptor CD95 and apoptosis-related genes. International Journal of Biological Macromolecules. 2018;119:1144-53
51. Zhang J-J, Gu M-M, Zheng T-T, Zhu J-J. Synthesis of Gelatin-Stabilized Gold Nanoparticles and Assembly of Carboxylic Single-Walled Carbon Nanotubes/Au Composites for Cytosensing and Drug Uptake. Anal Chem. 2009;81:6641-8
52. Morrone E, Sancey L, Dalonneau F, Ricciardi L, La Deda M. Conjugated Human Serum Albumin/Gold-Silica Nanoparticles as Multifunctional Carrier of a Chemotherapeutic Drug. IJMS. 2024;25:13701
53. Chen C-C, Li J-J, Guo N-H. et al. Evaluation of the Biological Behavior of a Gold Nanocore-Encapsulated Human Serum Albumin Nanoparticle (Au@HSANP) in a CT-26 Tumor/Ascites Mouse Model after Intravenous/Intraperitoneal Administration. IJMS. 2019;20:217
54. Di Natale C, Celetti G, Scognamiglio PL. et al. Molecularly endowed hydrogel with an in silico -assisted screened peptide for highly sensitive small molecule harvesting. Chem Commun. 2018;54:10088-91
55. Hamley IW. Protein Assemblies: Nature-Inspired and Designed Nanostructures. Biomacromolecules. 2019;20:1829-48
56. Di Natale C, La Manna S, Avitabile C. et al. Engineered $\beta$-hairpin scaffolds from human prion protein regions: Structural and functional investigations of aggregates. Bioorganic Chemistry. 2020;96:103594
57. Delfi M, Sartorius R, Ashrafizadeh M. et al. Self-assembled peptide and protein nanostructures for anti-cancer therapy: Targeted delivery, stimuli-responsive devices and immunotherapy. Nano Today. 2021;38:101119
58. Silva NHCS, Vilela C, Marrucho IM, Freire CSR, Pascoal Neto C, Silvestre AJD. Protein-based materials: from sources to innovative sustainable materials for biomedical applications. J Mater Chem B. 2014;2:3715
59. Wei G, Su Z, Reynolds NP. et al. Self-assembling peptide and protein amyloids: from structure to tailored function in nanotechnology. Chem Soc Rev. 2017;46:4661-708
60. Baghaienezhad M, Boroghani M, Anabestani R. Silver nanoparticles Synthesis by coffee residues extract and their antibacterial activity. Nanomed Res J. 2020 5
61. Wang J, Li Y, Nie G. Multifunctional biomolecule nanostructures for cancer therapy. Nat Rev Mater. 2021;6:766-83
62. Hong S, Choi DW, Kim HN, Park CG, Lee W, Park HH. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics. 2020;12:604
63. Du X, Zhou J, Shi J, Xu B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem Rev. 2015;115:13165-307
64. Yadav S, Sharma AK, Kumar P. Nanoscale Self-Assembly for Therapeutic Delivery. Front Bioeng Biotechnol. 2020;8:127
65. Yan J, Zhan X, Zhang Z. et al. Tetrahedral DNA nanostructures for effective treatment of cancer: advances and prospects. J Nanobiotechnol. 2021;19:412
66. Ghaemi Z, Gruebele M, Tajkhorshid E. Molecular mechanism of capsid disassembly in hepatitis B virus. Proc Natl Acad Sci USA. 2021;118:e2102530118
67. Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacological Reviews. 2017;69:200-35
68. Xing P, Zhao Y. Multifunctional Nanoparticles Self-Assembled from Small Organic Building Blocks for Biomedicine. Advanced Materials. 2016;28:7304-39
69. Kuan SL, Bergamini FRG, Weil T. Functional protein nanostructures: a chemical toolbox. Chem Soc Rev. 2018;47:9069-105
70. Williams DF. Biocompatibility pathways and mechanisms for bioactive materials: The bioactivity zone. Bioactive Materials. 2022;10:306-22
71. Zhu Q, Chen Z, Paul PK, Lu Y, Wu W, Qi J. Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharmaceutica Sinica B. 2021;11:2416-48
72. Coester C, Nayyar P, Samuel J. In vitro uptake of gelatin nanoparticles by murine dendritic cells and their intracellular localisation. European Journal of Pharmaceutics and Biopharmaceutics. 2006;62:306-14
73. Indikova I, Indik S. Highly efficient 'hit-and-run' genome editing with unconcentrated lentivectors carrying Vpr.Prot.Cas9 protein produced from RRE-containing transcripts. Nucleic Acids Research. 2020;48:8178-87
74. Gavas S, Quazi S, Karpiński TM. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res Lett. 2021;16:173
75. Kaltbeitzel J, Wich PR. Protein-based Nanoparticles: From Drug Delivery to Imaging, Nanocatalysis and Protein Therapy. Angew Chem Int Ed. 2023;62:e202216097
76. Rehman M, Khan A. Understanding the Interaction Between Human Serum Albumin and Anti-Bacterial/ Anti-Cancer Compounds. CPD. 2015;21:1785-99
77. Fang L, Shi C, Wang Y, Xiong Z, Wang Y. Exploring the diverse biomedical applications of programmable and multifunctional DNA nanomaterials. J Nanobiotechnol. 2023;21:290
78. Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Advanced Drug Delivery Reviews. 2018;130:73-89
79. Chubarov AS. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry. 2022;8:13
80. Saleh T, Soudi T, Shojaosadati SA. Aptamer functionalized curcumin-loaded human serum albumin (HSA) nanoparticles for targeted delivery to HER-2 positive breast cancer cells. International Journal of Biological Macromolecules. 2019;130:109-16
81. Mesken J, Iltzsche A, Mulac D, Langer K. Modifying plasmid-loaded HSA-nanoparticles with cell penetrating peptides - Cellular uptake and enhanced gene delivery. International Journal of Pharmaceutics. 2017;522:198-209
82. Luis De Redín I, Boiero C, Martínez-Ohárriz MC. et al. Human serum albumin nanoparticles for ocular delivery of bevacizumab. International Journal of Pharmaceutics. 2018;541:214-23
83. Kashyap BK, Singh VV, Solanki MK, Kumar A, Ruokolainen J, Kesari KK. Smart Nanomaterials in Cancer Theranostics: Challenges and Opportunities. ACS Omega. 2023;8:14290-320
84. Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. BioMed Research International. 2014;2014:1-12
85. Gulfam M, Kim J, Lee JM, Ku B, Chung BH, Chung BG. Anticancer Drug-Loaded Gliadin Nanoparticles Induce Apoptosis in Breast Cancer Cells. Langmuir. 2012;28:8216-23
86. Solanki PR. Gelatin Nanoparticles as a Delivery System for Proteins. JNMR. 2015 2
87. Li M, Luo Z, Zhao Y. Self-Assembled Hybrid Nanostructures: Versatile Multifunctional Nanoplatforms for Cancer Diagnosis and Therapy. Chem Mater. 2018;30:25-53
88. Lee D, Baek S, Kim Y-Y. et al. Self-Assembled DNA-Protein Hybrid Nanospheres: Biocompatible Nano-Drug-Carriers for Targeted Cancer Therapy. ACS Appl Mater Interfaces. 2022;14:37493-503
89. McNeale D, Esquirol L, Okada S. et al. Tunable In Vivo Colocalization of Enzymes within P22 Capsid-Based Nanoreactors. ACS Appl Mater Interfaces. 2023;15:17705-15
90. Habibi N, Kamaly N, Memic A, Shafiee H. Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery. Nano Today. 2016;11:41-60
91. Mao L, Wang H, Tan M, Ou L, Kong D, Yang Z. Conjugation of two complementary anti-cancer drugs confers molecular hydrogels as a co-delivery system. Chem Commun. 2012;48:395-7
92. Gholami A, Hashemi SA, Yousefi K. et al. 3D Nanostructures for Tissue Engineering, Cancer Therapy, and Gene Delivery. Li X, Ed. Journal of Nanomaterials. 2020;2020:1-24
93. El-Sawah AA, El-Naggar NE-A, Eldegla HE, Soliman HM. Green synthesis of collagen nanoparticles by Streptomyces xinghaiensis NEAA-1, statistical optimization, characterization, and evaluation of their anticancer potential. Sci Rep. 2024;14:3283
94. Lu Z, Yeh T-K, Wang J. et al. Paclitaxel Gelatin Nanoparticles for Intravesical Bladder Cancer Therapy. Journal of Urology. 2011;185:1478-83
95. Zhou L-Q, Li P, Cui X-W, Dietrich CF. Ultrasound nanotheranostics in fighting cancer: Advances and prospects. Cancer Letters. 2020;470:204-19
96. Wang L, Niu C. IR780-based nanomaterials for cancer imaging and therapy. J Mater Chem B. 2021;9:4079-97
97. Chang N, Lu S, Qin D. et al. Efficient and controllable thermal ablation induced by short-pulsed HIFU sequence assisted with perfluorohexane nanodroplets. Ultrasonics Sonochemistry. 2018;45:57-64
98. Ji Y, Lu F, Hu W. et al. Tandem activated photodynamic and chemotherapy: Using pH-Sensitive nanosystems to realize different tumour distributions of photosensitizer/prodrug for amplified combination therapy. Biomaterials. 2019;219:119393
99. Wang Y, Tang Y, Du Y. et al. Genetically engineered bacteria-mediated multi-functional nanoparticles for synergistic tumor-targeting therapy. Acta Biomaterialia. 2022;150:337-52
100. Song L, Hou X, Wong KF. et al. Gas-filled protein nanostructures as cavitation nuclei for molecule-specific sonodynamic therapy. Acta Biomaterialia. 2021;136:533-45
101. Ren J, Wu H, Lu Z. et al. Improved stability and anticancer activity of curcumin via pH-driven self-assembly with soy protein isolate. Process Biochemistry. 2024;137:217-28
102. Shah R, Petersburg J, Gangar AC, Fegan A, Wagner CR, Kumarapperuma SC. In Vivo Evaluation of Site-Specifically PEGylated Chemically Self-Assembled Protein Nanostructures. Mol Pharmaceutics. 2016;13:2193-203
103. Gil-Garcia M, Ventura S. Multifunctional antibody-conjugated coiled-coil protein nanoparticles for selective cell targeting. Acta Biomaterialia. 2021;131:472-82
104. Zhao T, Cai Y, Jiang Y. et al. Vaccine adjuvants: mechanisms and platforms. Sig Transduct Target Ther. 2023;8:283
105. Gabrielaitis D, Zitkute V, Saveikyte L. et al. Nanotubes from bacteriophage tail sheath proteins: internalisation by cancer cells and macrophages. Nanoscale Adv. 2023;5:3705-16
106. Palombarini F, Di Fabio E, Boffi A, Macone A, Bonamore A. Ferritin Nanocages for Protein Delivery to Tumor Cells. Molecules. 2020;25:825
107. Ashrafizadeh M, Delfi M, Zarrabi A. et al. Stimuli-responsive liposomal nanoformulations in cancer therapy: Pre-clinical & clinical approaches. Journal of Controlled Release. 2022;351:50-80
108. Garcia-Seisdedos H, Empereur-Mot C, Elad N, Levy ED. Proteins evolve on the edge of supramolecular self-assembly. Nature. 2017;548:244-7
109. Kuan SL, Ng DYW, Wu Y. et al. pH Responsive Janus-like Supramolecular Fusion Proteins for Functional Protein Delivery. J Am Chem Soc. 2013;135:17254-7
110. Zhu J, Avakyan N, Kakkis A. et al. Protein Assembly by Design. Chem Rev. 2021;121:13701-96
111. Wong SS, Wong L-JC. Chemical crosslinking and the stabilization of proteins and enzymes. Enzyme and Microbial Technology. 1992;14:866-74
112. Busseron E, Ruff Y, Moulin E, Giuseppone N. Supramolecular self-assemblies as functional nanomaterials. Nanoscale. 2013;5:7098
113. Hussain I, Singh NB, Singh A, Singh H, Singh SC. Green synthesis of nanoparticles and its potential application. Biotechnol Lett. 2016;38:545-60
114. Kandav G, Sharma T. Green synthesis: an eco friendly approach for metallic nanoparticles synthesis. Particulate Science and Technology. 2024;42:874-94
115. Poulose S, Panda T, Nair PP, Theodore T. Biosynthesis of Silver Nanoparticles. j nanosci nanotech. 2014;14:2038-49
116. Ying S, Guan Z, Ofoegbu PC. et al. Green synthesis of nanoparticles: Current developments and limitations. Environmental Technology & Innovation. 2022;26:102336
117. Shreyash N, Bajpai S, Khan MohdA, Vijay Y, Tiwary SK, Sonker M. Green Synthesis of Nanoparticles and Their Biomedical Applications: A Review. ACS Appl Nano Mater. 2021;4:11428-57
118. Razali Z, Norrizah JS, Abdullah S. Impact of temperature and pH on antioxidant activity of green silver nanoparticles fabricated from Ananas comosus peel extracts. IOP Conf Ser: Earth Environ Sci. 2022;1019:012006
119. Graham HN. Green tea composition, consumption, and polyphenol chemistry. Preventive Medicine. 1992;21:334-50
120. Balentine DA, Wiseman SA, Bouwens LCM. The chemistry of tea flavonoids. Critical Reviews in Food Science and Nutrition. 1997;37:693-704
121. Santhoshkumar J, Rajeshkumar S, Venkat Kumar S. Phyto-assisted synthesis, characterization and applications of gold nanoparticles - A review. Biochemistry and Biophysics Reports. 2017;11:46-57
122. Fahmy HM, Mohamed FM, Marzouq MH. et al. Review of Green Methods of Iron Nanoparticles Synthesis and Applications. BioNanoSci. 2018;8:491-503
123. Lee YJ, Ahn E-Y, Park Y. Shape-dependent cytotoxicity and cellular uptake of gold nanoparticles synthesized using green tea extract. Nanoscale Res Lett. 2019;14:129
124. Pourasgar S, Ranji N, Asadpour L, Shahriarinour M, Nikpassand M. Antibacterial and Anti-cancer Properties of Curcumin-Functionalized Silica-Coated Fe3O4 Magnetic Nanoparticles. Arab J Sci Eng. 2024
125. Shamaila S, Zafar N, Riaz S, Sharif R, Nazir J, Naseem S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials. 2016;6:71
126. Ashikbayeva Z, Bekmurzayeva A, Myrkhiyeva Z, Assylbekova N, Atabaev TSh, Tosi D. Green-synthesized gold nanoparticle-based optical fiber ball resonator biosensor for cancer biomarker detection. Optics & Laser Technology. 2023;161:109136
127. Al-Mafarjy SS, Suardi N, Ahmed NM, Kernain D, Hisham Alkatib H, Dheyab MA. Green synthesis of gold nanoparticles from Coleus scutellarioides (L.) Benth leaves and assessment of anticancer and antioxidant properties. Inorganic Chemistry Communications. 2024;161:112052
128. Bouttier-Figueroa DC, Loreto-Romero MA, Roldan MA. et al. Green synthesis of gold nanoparticles via Moringa oleifera seed extract: antioxidant, antibacterial and anticarcinogenic activity on lung cancer. Journal of Environmental Science and Health, Part A. 2024;59:231-40
129. Montazersaheb S, Eftekhari A, Shafaroodi A. et al. Green-synthesized silver nanoparticles from peel extract of pumpkin as a potent radiosensitizer against triple-negative breast cancer (TNBC). Cancer Nano. 2024;15:47
130. Sivakumar S, Subban M, Chinnasamy R. et al. Green synthesized silver nanoparticles using Andrographis macrobotrys Nees leaf extract and its potential to antibacterial, antioxidant, anti-inflammatory and lung cancer cells cytotoxicity effects. Inorganic Chemistry Communications. 2023;153:110787
131. Motafeghi F, Gerami M, Mortazavi P, Khayambashi B, Ghasemi-Barghi N, Shokrzadeh M. Green synthesis of silver nanoparticles, graphene, and silver-graphene nanocomposite using Melissa officinalis ethanolic extract: Anticancer effect on MCF-7 cell line. Iranian Journal of Basic Medical Sciences. 2023 26
132. Al Baloushi KSY, Senthilkumar A, Kandhan K. et al. Green Synthesis and Characterization of Silver Nanoparticles Using Moringa Peregrina and Their Toxicity on MCF-7 and Caco-2 Human Cancer Cells. IJN. 2024 Volume 19: 3891-905
133. Viswanathan S, Palaniyandi T, Shanmugam R. et al. Synthesis, characterization, cytotoxicity, and antimicrobial studies of green synthesized silver nanoparticles using red seaweed Champia parvula. Biomass Conv Bioref. 2024;14:7387-400
134. Jagaran K, Singh M. Copolymer-Green-Synthesized Copper Oxide Nanoparticles Enhance Folate-Targeting in Cervical Cancer Cells In Vitro. Polymers. 2023;15:2393
135. Majeed S, Saravanan M, Danish M. et al. Bioengineering of green-synthesized TAT peptide-functionalized silver nanoparticles for apoptotic cell-death mediated therapy of breast adenocarcinoma. Talanta. 2023;253:124026
136. Ren G, Hu D, Cheng EWC, Vargas-Reus MA, Reip P, Allaker RP. Characterisation of copper oxide nanoparticles for antimicrobial applications. International Journal of Antimicrobial Agents. 2009;33:587-90
137. Alishah H, Pourseyedi S, Ebrahimipour SY, Mahani SE, Rafiei N. Green synthesis of starch-mediated CuO nanoparticles: preparation, characterization, antimicrobial activities and in vitro MTT assay against MCF-7 cell line. Rend Fis Acc Lincei. 2017;28:65-71
138. Dadhwal P, Dhingra HK, Dwivedi V. et al. Hippophae rhamnoides L. (sea buckthorn) mediated green synthesis of copper nanoparticles and their application in anticancer activity. Front Mol Biosci. 2023;10:1246728
139. Sampath S, Sunderam V, Madhavan Y. et al. Facile green synthesis of zinc oxide nanoparticles using Artocarpus hirsutus seed extract: spectral characterization and in vitro evaluation of their potential antibacterial-anticancer activity. Biomass Conv Bioref. 2023
140. Mongy Y, Shalaby T. Green synthesis of zinc oxide nanoparticles using Rhus coriaria extract and their anticancer activity against triple-negative breast cancer cells. Sci Rep. 2024;14:13470
141. Dobrucka R, Długaszewska J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi Journal of Biological Sciences. 2016;23:517-23
142. Mageshwari K, Mali SS, Sathyamoorthy R, Patil PS. Template-free synthesis of MgO nanoparticles for effective photocatalytic applications. Powder Technology. 2013;249:456-62
143. Ali S, Sudha KG, Thirumalaivasan N. et al. Green Synthesis of Magnesium Oxide Nanoparticles by Using Abrus precatorius Bark Extract and Their Photocatalytic, Antioxidant, Antibacterial, and Cytotoxicity Activities. Bioengineering. 2023;10:302
144. Karthik K, Dhanuskodi S, Gobinath C, Prabukumar S, Sivaramakrishnan S. Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. Journal of Photochemistry and Photobiology B: Biology. 2019;190:8-20
145. Mosleh-Shirazi S, Kasaee SR, Dehghani F. et al. Investigation through the anticancer properties of green synthesized spinel ferrite nanoparticles in present and absent of laser photothermal effect. Ceramics International. 2023;49:11293-301
146. Askari N, Hojabrpour H, Mirzaei MR, Mirzaei V, Falahati-pour SK. Green Synthesis and Anti-Cancer Properties of Cerium Oxide Nanoparticles Using Pistachio Vera Pericarp Essential Oil. 2024.
147. Alhawiti AS. Citric acid-mediated green synthesis of selenium nanoparticles: antioxidant, antimicrobial, and anticoagulant potential applications. Biomass Conv Bioref. 2024;14:6581-90
148. Hamidian K, Zarin A, Sarani M, Barani M, Adeli-Sardou M. Study of cytotoxic performance of green-synthesized Co doped NiO nanoparticles over human breast cancer cells. Inorganic Chemistry Communications. 2024;162:112234
149. Nejad FS, Alizade-Harakiyan M, Haghi M. et al. Investigating the effectiveness of iron nanoparticles synthesized by green synthesis method in chemoradiotherapy of colon cancer. Heliyon. 2024;10:e28343
150. Dikshit P, Kumar J, Das A. et al. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts. 2021;11:902
151. Gaur J, Kumar S, Kaur H. et al. Eco-friendly innovation: harnessing nature's blueprint for enhanced photocatalysis and antimicrobial potential in multi-structured PN/ZnO nanoparticles. Funct Compos Struct. 2024;6:015005
152. Bolisetty S, Boddupalli CS, Handschin S. et al. Amyloid Fibrils Enhance Transport of Metal Nanoparticles in Living Cells and Induced Cytotoxicity. Biomacromolecules. 2014;15:2793-9
153. Mohammadian M, Waly MI, Moghadam M, Emam-Djomeh Z, Salami M, Moosavi-Movahedi AA. Nanostructured food proteins as efficient systems for the encapsulation of bioactive compounds. Food Science and Human Wellness. 2020;9:199-213
154. Anselmo S, Fricano A, Sancataldo G, Vetri V. Sustainable Formation of Gold Nanoparticle-Decorated Amyloid Fibrils for the Development of Functional Hybrid Materials. Langmuir. 2025;41:172-83
155. Veetil VT, Jayakrishnan V, Aravindan V, Rajeeve AD, Koolath S, Yamuna R. Biogenic silver nanoparticles incorporated hydrogel beads for anticancer and antibacterial activities. Sci Rep. 2024;14:27269
156. Beg M, Maji A, Nayim S. et al. Biophysical insights into the interaction of human serum albumin with Cassia fistula leaf extracts inspired biogenic potent antibacterial and anticancerous gold nanoparticles. Journal of Biomolecular Structure and Dynamics. 2021;39:4567-81
157. Basu A, Ray S, Chowdhury S. et al. Evaluating the antimicrobial, apoptotic, and cancer cell gene delivery properties of protein-capped gold nanoparticles synthesized from the edible mycorrhizal fungus Tricholoma crassum. Nanoscale Res Lett. 2018;13:154
158. Amina SJ, Guo B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. IJN. 2020 Volume 15: 9823-57
159. Kapoor D, Maheshwari N, Soni N. et al. Metallic nanoparticles in cancer: Types, green synthesis, applications, tumor microenvironment and toxicity considerations. Journal of Drug Delivery Science and Technology. 2024;92:105307
160. Liu H, Liu X, Jiang H, Wang X. Design Principles Of Inorganic-Protein Hybrid Materials for Biomedicine. Exploration. 2025;5:20240182
161. Qin Y, Chen X, Willner I. Nucleic Acid-Modified Nanoparticles for Cancer Therapeutic Applications. Small. 2025;21:2500843
162. Wang R, Zhang L, Razzaq A. et al. Albumin-coated green-synthesized zinc oxide nanoflowers inhibit skin melanoma cells growth via intra-cellular oxidative stress. International Journal of Biological Macromolecules. 2024;263:130694
163. Shanahan K, Coen D, Nafo W. Polymer-based nanoparticles for cancer theranostics: advances, challenges, and future perspectives. Explor BioMat-X. 2025;2:101342
164. Morgan RN, Aboshanab KM. Green biologically synthesized metal nanoparticles: biological applications, optimizations and future prospects. Future Science OA. 2024;10:FSO935
165. El-Seedi HR, Omara MS, Omar AH. et al. Updated Review of Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. Bioengineering. 2024;11:1095
166. Ilavenil KK, Senthilkumar V, Kasthuri A. Green synthesis of metal nanoparticles from three medicinal plants: a review of environmental and health applications. Discov Catal. 2025;2:3
167. Nyandoro VO, Masioge HK, Malago ZL. Biogenic synthesis of metal nanoparticles: promoting green nanotechnology and sustainable development goals. Clean Techn Environ Policy. 2025;27:517-30
168. Kirubakaran D, Wahid JBA, Karmegam N. et al. A Comprehensive Review on the Green Synthesis of Nanoparticles: Advancements in Biomedical and Environmental Applications. Biomedical Materials & Devices. 2026;4:388-413
169. Kumar S, Yadav I, Aswal VK, Kohlbrecher J. Structure and Interaction of Nanoparticle-Protein Complexes. Langmuir. 2018;34:5679-95
170. Harun-Ur-Rashid M, Jahan I. Innovative hybrid nanostructures: pioneering advances in modern therapy. Front Nanotechnol. 2024;6:1458894
171. Macchione M, Biglione C, Strumia M. Design, Synthesis and Architectures of Hybrid Nanomaterials for Therapy and Diagnosis Applications. Polymers. 2018;10:527
172. Taylor-Pashow KML, Della Rocca J, Huxford RC, Lin W. Hybrid nanomaterials for biomedical applications. Chem Commun. 2010;46:5832
173. Dr K Vijaya Lakshmi. Artificial Intelligence and its Applications in Nanochemistry. IJETMS. 2023;7:385-9
174. Singh AV, Rosenkranz D, Ansari MHD. et al. Artificial Intelligence and Machine Learning Empower Advanced Biomedical Material Design to Toxicity Prediction. Advanced Intelligent Systems. 2020;2:2000084
175. Cassano A, Robinson RLM, Palczewska A. et al. Comparing the CORAL and Random Forest Approaches for Modelling the In Vitro Cytotoxicity of Silica Nanomaterials. Altern Lab Anim. 2016;44:533-56
176. Baharifar H, Amani A. Cytotoxicity of chitosan/streptokinase nanoparticles as a function of size: An artificial neural networks study. Nanomedicine: Nanotechnology, Biology and Medicine. 2016;12:171-80
177. Choi J-S, Ha MK, Trinh TX, Yoon TH, Byun H-G. Towards a generalized toxicity prediction model for oxide nanomaterials using integrated data from different sources. Sci Rep. 2018;8:6110
178. Gerloff K, Landesmann B, Worth A, Munn S, Palosaari T, Whelan M. The Adverse Outcome Pathway approach in nanotoxicology. Computational Toxicology. 2017;1:3-11
179. Schmid AJ, Dubbert J, Rudov AA. et al. Multi-Shell Hollow Nanogels with Responsive Shell Permeability. Sci Rep. 2016;6:22736
Author contact
![]() Corresponding author: Ravindra Kumar, Email: ravindraac.in; Tel.: +91 0495-2285475; ORCID: 0000-0001-5577-9048.
Corresponding author: Ravindra Kumar, Email: ravindraac.in; Tel.: +91 0495-2285475; ORCID: 0000-0001-5577-9048.

 Global reach, higher impact
Global reach, higher impact