ISSN: 2206-7418
Nanotheranostics 2025; 9(2):186-198. doi:10.7150/ntno.109280 This issue Cite
Research Paper
Doxorubicin-NFL-TBS.40-63 peptide Gold Complex Nanovector (DOX IN-NFL@AuNPs): Efficacy Evaluation in a mouse transplantation tumor model induced by PANC-1/ADR human pancreatic cancer resistant strain cells
1. Department of Hepatobiliary Surgery, Guangdong Provincial Key Laboratory of Regional Immunity and Diseases & Carson International Cancer Center, Shenzhen University General Hospital & Shenzhen University Clinical Medical Academy Center, Shenzhen University, Shenzhen, 518083 China.
2. CNRS, UMR 7244, NBD-CSPBAT, Laboratoire de Chimie, Structures et Propriétés de Biomatériaux et d'Agents Thérapeutiques Université Paris 13, Sorbonne Paris Nord, Bobigny 93000, France; University Sorbonne Paris Nord; CB3S, UMR CNRS 7244, Insitut Galilée, 99 France.
3. Laboratoire Micro et Nanomédecines Translationnelles, Inserm 1066, CNRS 6021, Institut de Recherche en Ingénierie de la Santé, Bâtiment IBS Institut de Biologie de la Santé, Université d'Angers, Centre Hospitalier Universitaire, Angers, 49100 France.
‡ These authors contributed equally to this work.
Received 2024-12-23; Accepted 2025-3-27; Published 2025-6-19
Abstract
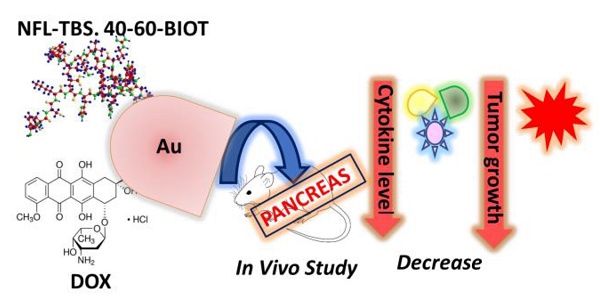
The key role of the NFL-TBS.40-63 peptide (BIOT-NFL) is to target and destroy glioma cancer cells. Recently we have performed a novel peptide-hybrid-gold nanovector (BIOT-NFL-PEG-AuNPs) capable to destroy microtubule network of pancreatic cancer cells (PDAC) exhibiting a decrease of tumor index with a real anti-angiogenic effect. In order to improve the scientific background of our study, we conceived a chemotherapeutic hybrid nanovector based on gold-doxorubicin (DOX) functionalized with the NFL-TBS.40-63 peptide (BIOT-NFL) as a promising therapeutic in PDAC cancer. Mouse transplantation tumor model induced by PANC-1/ADR human pancreatic cancer resistant strain cells, was used to evaluated the therapeutic efficacy of DOX IN-NFL@AuNPs as chemotherapeutic nano-drug. Our results indicate that DOX IN-NFL@AuNPs have a great impact on the decrease of the tumor growth and decreased the tumor index with a relevant effect on cytokines and ROS levels, thus confirming the impact of DOX IN-NFL@AuNPs to boost the immune system.
Keywords: BIOT-NFL peptide, Doxorubicin, Gold Complex, PDAC, immune system.
Introduction
Pancreatic Ductal Adeno Carcinoma (PDAC) has one of the highest mortality rates of any cancers1. No efficient treatments have been found for improving the prognosis and reducing the deaths of patients due to PDAC. Typically for the late-stage diagnoses limit successful surgical resections, with 80-85% being unresectable at the time of diagnosis2. The alternative therapies could only be relied on the chemotherapeutics such as doxorubicin, gemcitabine and paclitaxel3,4,5, nevertheless, the difficulty for chemotherapy treatment of PDAC specially link to the presence of a dense desmoplastic reaction (DR)6 which consists a largely portion of fibroblasts, pancreatic stellate cells (PSCs), and extracellular matrix (ECM) proteins, including collagens I and III and fibronectin7,8. In another hand, the stroma9,10 includes endothelial cells, immune cells, pericytes, and nerve fibers. All these factors create a solid barrier preventing the conventional chemotherapeutics penetrate inside the PDAC (chemo-resistance). Some of the widely used chemotherapeutics, as doxorubicin, have been suggested to induce anti-tumor immunity through the stimulation of immunogenic cell death (ICD)11. Recent research shows that the cytotoxic chemotherapy induces the generation of anti-cancer immunity and durable tumor responses, which is considered as a promising method to treat cancer 12-14.
To increase the durability of anti-cancer immune responses, both antigen recognition and adjuvant signals result from cell stress or death were required15,16. Immune-stimulating chemotherapies incite the release of pro-inflammatory signals, including damage-associated molecular patterns (DAMPs), that indicate danger and act as immunologic adjuvants, provoking anti-tumor immunity. However, even in settings where tumor antigens are present, cytotoxic chemotherapy rarely generates durable anti-cancer immune responses. This suggests that any immune stimulus from genotoxic therapy is insufficient or ultimately suppressed62. To overcome the drug resistance, lasting the durability of cytotoxic chemotherapy in terms of antigen recognition and adjuvant signals for PDAC treatment, it is urgent to find some new alternatives, hence drug delivery systems (DDSs) could be considered as an efficacious system17. Different nanocarriers such as dendrimers18,19, liposomes20, metal nanoparticles21, polymer micelles and vesicles22,23 have been utilized to improve the targeted delivery of the therapeutics for cancer treatment.
Among all the DDSs,the application of gold nanoparticles (AuNPs) is growing field24,25. The inherent, non-toxic and biocompatible properties make them a promising vehicle for drug delivery. Therefore the biomolecule-conjugated gold nanoparticles are considered as the promising candidate in our studies due to its surface properties which can be tuned by using biomolecules, such as, antibodies and peptides26. The NFL-TBS.40-63 peptide has been conceived by J. Eyer and his team 31-33. This peptide interacts specifically in several glioblastoma cell lines reducing glioblastoma viability through the blood-brain barrier inducing the inhibition of the formation of microtubules34. NFL-TBS.40-63 peptide is also internalized massively in glioblastoma cells and poorly in other cells from the nervous system, like astrocytes and neurons. Recently, we have compared the power of NFL-TBS.40-63 peptide onto pancreatic cancer cells; for this aim we synthetized a gold-complex biotinylated NFL-TBS.40-63 (BIOT-NFL) to form a hybrid gold nanovector (BIOT-NFL-PEG-AuNPs), by methodology IN discovered by J. Spadavecchia et al.27-30.
It was demonstrated for the first time that BIOT-NFL-PEG-AuNPs have the ability to target the destruction of pancreatic cancer cells (PDAC) under in vivo experimental conditions, by varying the metabolic profiles of these MIA-PACA-2 cells27.
It is known that the PDAC response to stiffness and solid stress in pancreas, is driven by the change in ECM-Cell interaction, influenced mainly by the behavior of the micro-fluidic conditions. With those new controlled peptide structure and conditions, we are presenting simultaneously in the future study, a new multi-physics concept to base a proof of concept and hypothesis of computational analysis in pancreatic complex network, enhancing the reverse impact of the PDAC on the ECM, and controlling the micro-fluidic conditions change to promote peptides pathways and strength, with a customized potential. By generating a non-favorable ECM platform for tumor growth, a biomechanics effects destroys molecular messengers and Tumor growth factor, like β (TGF-β) or Sonic hedgehog (SHH) altering ECM deposition by cancer-associated fibroblasts (CAFs), compromising the pro-tumorigenic programs.
Encouraged by our previous results, we have decided to integrate chemotherapeutic doxorubicin in nanocomplex to formulate a new advanced nanoformulation (DOX IN-NFL@ AuNPs). To estimate the therapeutic efficacy of DOX IN-NFL@AuNPs, a mouse transplantation tumor model induced by PANC-1/ADR Human Pancreatic Cancer Resistant Strain Cells was applied. Several in vivo studies included different concentrations of DOX and BIOT-NFL into nanovectors have been carried out and the relevant results show that DOX IN-NFL@ AuNPs have a high impacted on the tumor growth and consequently decreased the tumor index without change body weight of mice. An excellent induction of ROS reaction, confirms the ability of DOX IN-NFL@ AuNPs to alter the metabolic profiles of PANC-1/ADR Human Pancreatic Cancer Resistant Strain Cells. The cytokine levels were also detected to evaluate the effect of serum inflammatory factors and the power of DOX IN-NFL@ AuNPs to boost the immune system.
We assume that this study is very important to provide a newly advanced PDAC peptide-chemotherapeutic therapy which could overcome the limits and poor effects of the classic chemotherapeutic drugs for PDAC therapy due to drug resistance following desmoplastic stroma. In the further study, as initiated previously, the new concept of micro-fluidic potential spread, driven by the change of ECM-Cell micro-environment and stiffness, will leverage the capability and the nutritional factors of the tumor progress. One field of application of this new hypothesis computational model including the new peptides described in this study, will permit to control the growth of the metastases process. Having a growth tumor endothelial remodeling, driven by tumor migrating compounds (TMC) like CTC and EV's expanding the metastases. Combine the provided numerical output data, with the controlled structure, the new nano-formulation and the physical conditions of the DOX IN-NFL@AuNPs, permit to well customize the injection rate locations and pathways, and target the reinforcement spread of the electrical potential by adapting its Zeta potential, in order to build a specified ECM and virtual layers of Basements Membrane (BM) around the tumor cellular matrix. The aim is to maintain a targeted environment surrounding initiated PDAC deposition, promoting tumor vessel collapse and osmolarity hijacking. In other hand, the new model and concept will support the peptides to use the ECM and BM compounds and tissue matrix organization, to control the environment physical condition by generating a targeted spinning flow, changing micro-fluidic viscosity and shear stress. This configuration creates a sever micro-environment with thresholds well above the resistance of the TMC membrane, hence, destroying and disrupting metastatic pockets.
Methodology
Chemical design and conception of gold nanovector (DOX IN-NFL@AuNPs)
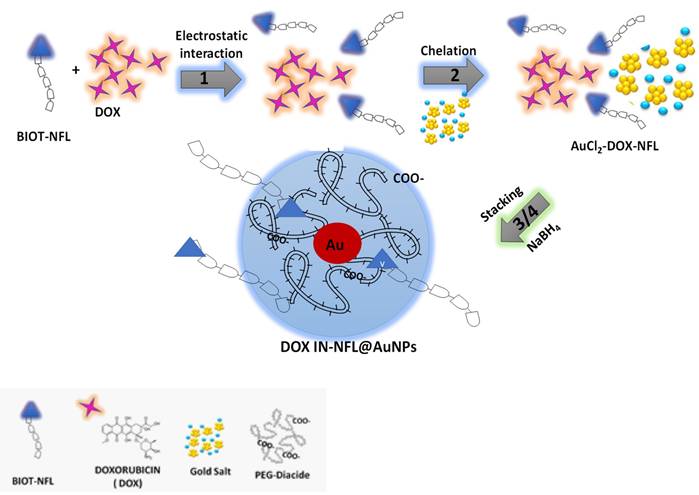
J. Spadavecchia et al. have exhaustively studied the chemical mechanism of complexation methodology (Method IN) with a large library of drugs, biomolecules (proteins, enzymes, aptamers, biological cofactors) as stabilizers and/or reagents on the hybrid- nanoparticles30-35. Recently, the same authors have designed and protected, the encapsulation of biotinylated cell penetrating peptide (CPP) as hybrid theragnostic complexes and their biological activity (P7391FR00-50481 LIV)27 35 36. In the present study, we developed a novel hybrid gold nanovector in which biotinylated-NFL and DOX participate actively in the grow process to obtain hybrid gold nanovectors (DOX IN-NFL@AuNPs) chemically stable and biological efficient.
The formation of DOX IN-NFL@AuNPs comprehends the chemical steps depicted in Scheme 1:
(1) Electrostatic interaction between biotinylated peptide NFL-TBS-60-43 (BIOT-NFL) and Doxorubicin (DOX) to generate hybrid-DOX-peptide (DOX-NFL),
(2) Chelation between DOX -NFL complex and gold salt (AuCl4-) to form hybrid-DOX-peptide (DOX-NFL) gold cluster28 30 (AuCl2-DOX-NFL),
(3) Stacking process of biocompatible polymer (PEG-Diacide) onto hybrid-DOX-peptide (DOX-NFL) gold cluster (AuCl2-DOX-NFL),
(4) Complete reduction and formation of hybrid-gold nanovector (DOX IN-NFL@AuNPs).
In the first step, the biotinylated peptide NFL-TBS-60-43 (BIOT-NFL) and doxorubicin (DOX) were mixed and interacts through the positively charged amino (-NH3+) of DOX and carboxylic group (COO-) of peptide in water solution, producing an electrostatic complex (DOX-NFL). In the second step, the obtained DOX-NFL interacts with a gold salt solution (HAuCl4), thanks to the chelation between hydroquinone-quinone and gold as discussed previously28 30. The further addiction of the polymer (PEG diacide) onto hybrid-DOX-peptide (DOX-NFL) gold cluster (AuCl2-DOX-NFL), promotes the kinetics of reduction by chelation with the Au ions21 and the colloidal stabilization after reduction process in the final step.
Results and Discussion
Spectroscopic Evaluation
As followed in Figure 1A, black line, the UV-Vis spectra of the DOX IN-NFL@AuNPs, exhibits a small peak at 295 nm and a characteristic peak at 215 nm responsible to π-π* electronic transitions associated to peptide's backbone.
The electrostatic interaction between BIOT-NFL-peptide and DOX followed by complexation to gold salt to form DOX-NFL-AuCl2-, display two peaks at 230-242 nm and a characteristic peak at 312 nm due to gold salt complex (Figure 1-A, blue line). After polymer stacking step (AuCl2-DOX-NFL-PEG diacide) we observed a strong decrease of the peak at 312 nm and the peak at 218 nm (Figure 1A, red line) with appearance of small peak at 480 nm 30 due to the formation of gold clusters ascribed to reduction started by carboxylic groups of PEG30. This spectroscopic fingerprint was associated to π-π* electronic transitions between the NFL-peptide backbone and gold salt ions, with a variation of the steric arrangement of peptide under gold-salt complex and migration through PEG molecules as discussed previously36. Finally, the reduction mixture with NaBH4 reduces completely gold species from AuIII to Au0 to form DOX IN-NFL@AuNPs (Figure 1A, black line).
Schematic design of the chemical methodology to obtain DOX IN-NFL@AuNPs (Method IN).
A) UV-Vis absorption of DOX IN-NFL@AuNPs (black line); each step of gold complex formation was carried out: AuCl2- DOX-NFL (blue line); DOX-NFL-AuCl2-PEG Diacide (red line); B) TEM images of DOX IN-NFL@AuNPs. Scale bars: 100 nm.
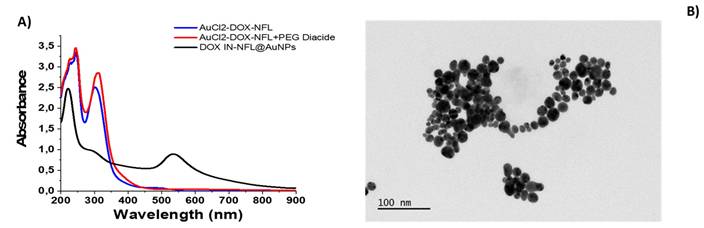
TEM images of DOX IN -NFL@AuNPs display a polydisperse nanoparticles with an average size of 15.52 ± 4.4 nm obtained by Image J software (Particle Analysis (imagej.net); ImageJ) onto each Image TEM (Figure 1-B). The hydrodynamic diameter measured by DLS technique is about 85 ± 2 nm due to steric arrangement of polymer onto gold peptide complex with a Zeta potential of -35.7 mV (data showed in Supporting information). As proved previously, the presence of biotin onto peptide improves a better chemical and steric configuration and consequently active targeting on cancer cells.
In vivo antitumor efficacy
Biomolecules targeting microtubules are extensively applied in cancer therapy with a good efficacy38. However, their administration provokes several secondary effects. Thanks to the advancement of research and knowledge on the structure of tubulin, a better comprehension of the mechanism of action provided the development of original therapeutic approaches39.
Doxorubicin (DOX) is an anthracycline with a chemical structure well defined and studied with high activity in cancer therapy40. The major issue has been the Doxorubicin (DOX), conferring drug resistance41,42. Whereas DOX-conjugated NPs have been used as drug carrier and delivery platform especially in treating several human cancers43,44, their use in the treatment of human pancreatic cancer cells has been little explored. Since 2016 Spadavecchia et al. promoted a novel methodology in which drugs and/or biomolecules were chelated to gold salt to form hybrid nanovector. In this steric and chemical conformation, we obtained excellent results in terms of efficacy in vitro and in vivo28,30,45,46.
As discussed previously, J. Eyer and co. have analyzed a peptide which sequence on the neurofilament light subunit (NFL-TBS.40-63) is capable to fix tubulin dimers on specific sites13 and can inhibit the proliferation of glioma cells by destroying their microtubule network38.
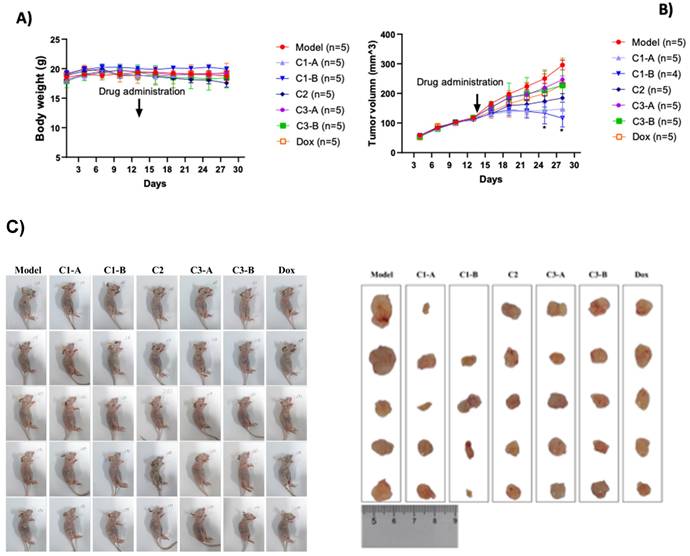
Recently, we realized a gold nano formulation composed by biotinylated-NFL-TBS.40-63 peptide (BIOT-NFL) complexed to gold salt and pegylated chains (BIOT-NFL-PEG-AuNPs) and we have studied their power of internalization on PDAC cells (P7391FR00-50481 LIV) including the capacity of the BIOT-NFL-PEG-AuNPs to target mouse transplantation tumor model PDAC improving their anti-cancer efficacity38. Herein we study the dual therapeutic effect of DOX as chemotherapeutic agent and NFL-TBS.40-63 peptide (BIOT-NFL) as gold nanoformulation depicted in Table 1, under some experimental conditions38. The therapeutic effect of DOX IN-NFL@AuNPs was monitored using mouse transplantation tumor model under hypodermic injection as shown in Figure 2A. All nanomaterials (C1-A-C3-B) did not significantly affect the body weight of PANC-1/ADR ruffed nude mice. We also observed a reduction of tumor (Figure 2B). The results of white light plots, tumor volume curves and endpoint tumor volume sizes of tumor-bearing nude mice in each group are shown in Figure 2B -2C. Indeed, at the end of the experiment, the nude mice in each group were dissected and the tumor and each organ were weighed to calculate the tumor and organ indices (Figure S1 in Supporting Information). As shown in Figure S1 in Supporting Information, C1-A and C1-B could reduce the tumor indices, and there was not significant difference in the organ indices of the remaining groups.
C1-B more significantly reduced the tumor volume of pancreatic cancer model mice (p < 0.05) (Figure 2B). On the 28th and 31st days and meanwhile the model group (PANC-1/ADR) significantly reduced the tumor size of pancreatic cancer mice as well (p < 0.05).
The presence of DOX in gold nanoparticles confers a different steric stability of the peptide allowing the destruction of microtubules.
ROS evaluation
It was established that doxorubicin-induced ROS over production occurs inside mitochondria and is mediated by the mitochondrial NADPH oxidase activity47. Previously we compared the effect of free DOX, before and after encapsulation in polymer gold complex28 30. Following this methodology30, DOX was chelated to gold salt previous deprotonation of C24 before interaction to polymers chains. In this steric conformation, DOX chelated to gold complex do not bind Fe3+/Fe2+ and so do not promote ROS production responsible of cardiotoxicity. Previously, we evaluated the biological effect of the NFL-TBS 60-43 peptide on glioblastoma and pancreatic cell mitochondria48. Others authors indicated that NFL can interact directly with mitochondria, such as vimentin, to regulate mitochondrial motility interacting with a N-terminal domain of vimentin49. Based on previously studies, we have demonstrated the exceptional activity of a combined formulation composed by NFL-TBS.40-63 peptide under biotinylated form and doxorubicin into gold complex nanoparticles (DOX IN-NFL@AuNPs) to PDAC.
Our studies showed that ROS can oxidatively damage tumor cells and improve tumor cell apoptosis. The experimental results are shown in Figure 3, compared with the model group, the C1-A and C1-B groups could significantly increase the ROS content in the tumor cells of mice with pancreatic cancer (p < 0.0001). C1-A group (DOX IN-NFL@AuNPs diluted 4 times) have a better effect compared to C1-B group (DOX IN-NFL@AuNPs diluted 2 times) and C3-A/C3-B groups corresponding to BIOT-NFL at specific concentrations.
Animal grouping and drug administration.
| Group | Drug | Quantity | Administration of injection | Administration frequences |
|---|---|---|---|---|
| C1-A | DOX IN-NFL@AuNPs working solution diluated 4 times | 5 | i.v. 1.25 mg/ml | 3/week |
| C1-B | DOX IN-NFL@AuNPs working solution diluated 2 times | 5 | i.v. 1.25 mg/ml | 3/week |
| C2 | DOX-IN working solution diluated 2 times | 5 | i.v. 2.5 mg/ml | 3/week |
| C3-A | NFL (Equal dose to group C1-A) | 5 | i.v. | 3/week |
| C3-B | NFL (Equal dose to group C1-B) | 5 | i.v. | 3/week |
| DOX | DOX | 5 | i.v. | 3/week |
A) Effect of DOX IN-NFL@AuNPs on the body weight of pancreatic cancer in mice. Body weight was shown as Mean ± SD by Two-way ANOVA. Model group compared with DOX IN-NFL@AuNPs group, *p<0.05 and **p<0.05. B) DOX IN-NFL@AuNPs on the tumor volume of pancreatic cancer in mice. Tumor volume (mm3). C) Representation of tumor volume sizes of tumor-bearing nude mice in each group.
Statistical analysis of DOX IN-NFL@AuNPs (C1-A-C1-B groups) and model groups as reference (Model, DOX, C2; C3-A; C3-B) on the ROS content of PANC-1 cancer cell after i.v. onto mice. Data was shown as Mean ± SD. Data was analyzed by One-way ANOVA, *p<0.05, **p<0.01 and ***p<0.001.
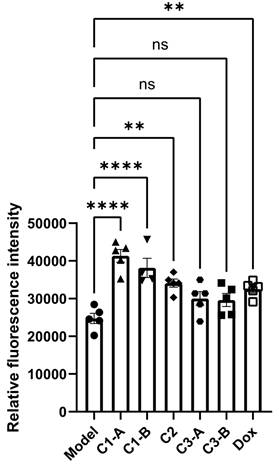
We also estimate that C2 group (DOX IN PEG AuNPs) and DOX group (free DOX) both impacts the ROS content in pancreatic cancer cells with similar results. Accordingly of these findings, we can conclude that the combination of DOX and BIOT-NFL at specific doses, confers a better steric and chemical configuration of the complex that improves ROS content in pancreatic cancer cells.
Blood screening
The number of cells in the whole blood of nude mice was detected using a hemocytometer.
Hematology screening is a relevant analysis to check a good response after chemotherapy treatment in oncology field, and the state of inflammation50. As proved previously, several parameters connected to red blood are altered by anthracycline drugs as doxorubicin and other metabolites with consequent alteration of membrane structure and biochemical process of metabolism51. Contrarily to chemotherapeutic drugs (i.e. doxorubicin), we demonstrated in previous study that NFL- TBS 60-53 under gold nanoformulation (BIOT-NFL-PEG-AuNPs)27 improve the number of WBC, NE and LY (p<0.01), and significantly reduce the content of MCV and MCH (p < 0.01), with an outstanding effect on platelets production27. Herein, we discovered that a dual combination of DOX and NFL-TBS 60-53 (BIOT-NFL) into gold formulation did not affect the number of blood cells compared to the model group.
All experimental results are shown in Figure S2 in Supporting Information.
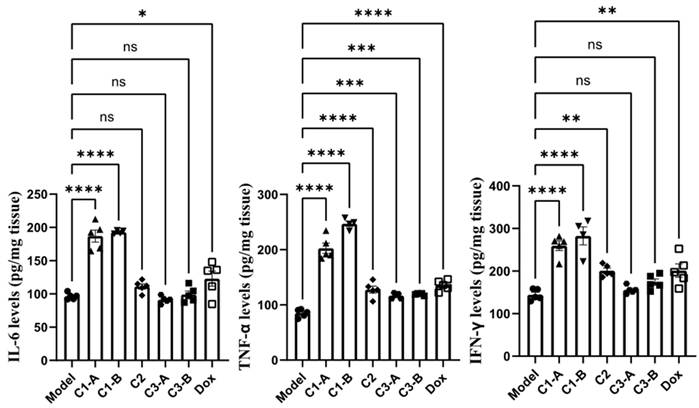
Effect of DOX IN-NFL@AuNPs on IL-6, TNF-α, IFN-γ as pro-inflammatory cytokines
The pro-inflammatory cytokine, plays a central role in oncogenesis, cancer progression, invasiveness, microenvironment changes, treatment resistance and prognosis52. Cytokines including IL-6, IFN-γ and TNF-α have been monitored in pre-clinically in vitro and in vivo, studies playing a key role in immunotherapy and cancer diseases53. These cytokines, participate into regulation of immune cell proliferation and the decrease of tumor growth showed in pre-clinical tests in vitro and in vivo. TNF-α is involved in the host immune response and systemic inflammation54, playing a key role in apoptotic cell and tumor necrosis, confirming an excellent anti-tumor activity55. IL-6 is a cytokine also implicated in the regulation of several processes of immune system47, improving the proliferation of responsive T-cells. TNF-α has been establish to play a key role in anti-tumor activity in order to induce apoptotic cell death and tumor necrosis56. In addition, previous studies have demonstrated clear evidence that IFN-γ promoted specific immune responses, through immunological processes on the growth of tumor cells57. On the other way, IFN-γ is used as adjuvant for immunotherapy in several types of cancer. Besides, IFN-γ inhibits angiogenesis in tumor tissue, induces regulatory T-cell apoptosis, stimulating the activity of macrophages, confirming a key role in tumor progression58.
In the previous study we analyzed a large panel of cytokines with a great improvement onto cytokines by NFL peptide under gold nanoformulation27.
Herein, we combined and studied a dual effect of DOX and NFL after injection of DOX IN-NFL@AuNPs (C1-A-C1-B groups) onto PDAC tumor-bearing mice, discovering that the levels of serum IL-6, IFN-γ and TNF-α (p<0.05) were strongly improved. Compared to the model group, C1-B can significantly increase the content of TNF α and IL-6 in serum and tumor tissues (p < 0.05); C1-A group can significantly increase the content of IFN γ in serum and tumor tissues (p < 0.05) (Figure 4). These results confirm that the different doses of DOX and NFL peptide in the gold nanoformulation influence the biological behavior and the impact on the immunity system.
Biodistribution and histological evaluation
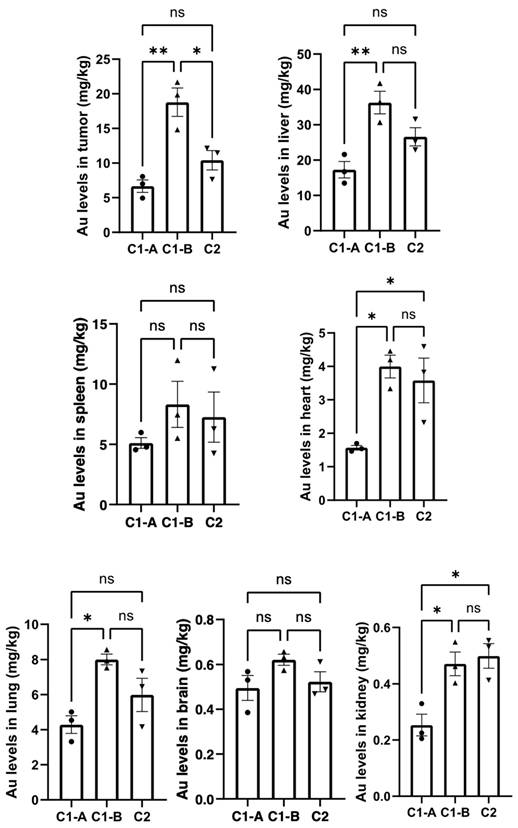
The distribution of gold nanoparticles in each group of organs was detected by ICP-MS after administration27, and the experimental results are shown in Figure 5. In all tissues, the content of gold nanoparticles in group C1-B was higher than that in groups C1-A and C2.
DOX IN-NFL@AuNPs cytokine effect on the content of inflammatory factors in pancreatic cancer mice. Data was shown as Mean ± SD. The data was analyzed by One-way ANOVA, *p<0.05, and **p<0.01.
Higher distribution of nanomaterials in tumor tissue of pancreatic cancer mice. Data was shown as Mean ± SD. The data was analyzed by One-way ANOVA, *p<0.05, **p<0.01, ***p <0.001 and ****p<0.0001.
When nanoparticles are administrated through intravenous system, a large amount of them is stored in the liver. In previous study we showed that the pegylated nanoparticles decorated with NFL-TBS-60-43 (BIOT-NFL) reduce their adsorption in the organs with a strong reduction in the risk of long-term toxicity27.
Herein, we have verified the synergic role of DOX and BIOT-NFL in gold nanoformulation after intravenous administration. As showed in Figure 5, we observed a great accumulation of DOX IN-NFL@AuNPs at fixed dose (C1-B) in tumor heart and liver. Contrarily, the form C1-A decrease in the liver, due to the presence of DOX at different concentration.
However, the presence of DOX IN-NFL@AuNPs (C1-B) in the lung and kidney confirms the lack of translocation into the circulatory system and secondary organs59. We confirmed an excellent accumulation of DOX IN-NFL@AuNPs (C1-B) in the brain that this is crucial to validate the synergic role of our nanovector. These results also suggest that DOX IN-NFL@AuNPs improved lung bioavailability to control excessive inflammation. Furthermore, the rapid excretion of nanoparticles via the kidney could reduce their risk of long-term toxicity. This indicates a reduction in the risk of long-term toxicity.
We can assume that our system DOX IN-NFL@AuNPs (C1-B) showed a good metabolic profile to control the acute inflammation.
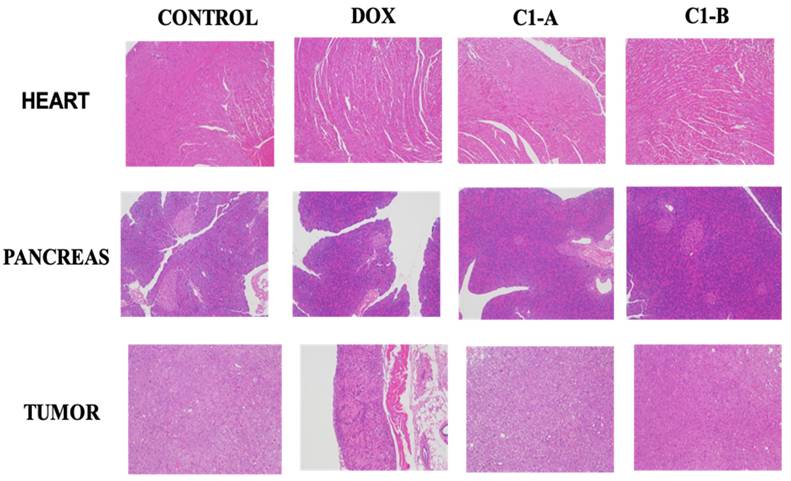
As proved previously, DOX is capable to modify iron metabolism triggering ROS production responsible of cardiotoxicity and cardiomyopathy60. To evaluate the chronic myocardial toxicity of DOX before and after complexation to nanovector, a group of mice was treated with free DOX and DOX IN-NFL@AuNPs under two forms (C1-A; C1-B) and then analyzed by histology after the last administration. The heart tissue sections from the free DOX group exhibited strong myocardial pathological changes with the presence of characteristic myocardial fibers with several degrees of rupture28 (Figure 6). After treatment with our nanovector (DOX IN-NFL@AuNPs) (C1-A; C1-B), we observed a disappearance of myocardial fibers confirming the efficacity of our system. In particular we assumed a better result with C1-B, confirming the key role of the dose, chemical-steric arrangement and consequent therapeutic effect. As discussed previously28 DOX-gold complex significantly eliminated the chronic myocardial toxicity of DOX during the period of treatment. Indeed, in our methodology, DOX was complexed to BIOT-NFL and gold salt by chelation30. In this chemical conformation, DOX does not bind Fe3+/Fe2+, and does not stimulate the ROS production that is responsible for cardiotoxicity28. As illustrated in Figure 5, no abnormalities were also observed in the main organs as pancreas and tumor. These results indicated the good tolerance and biosafety of all treatment formulations.
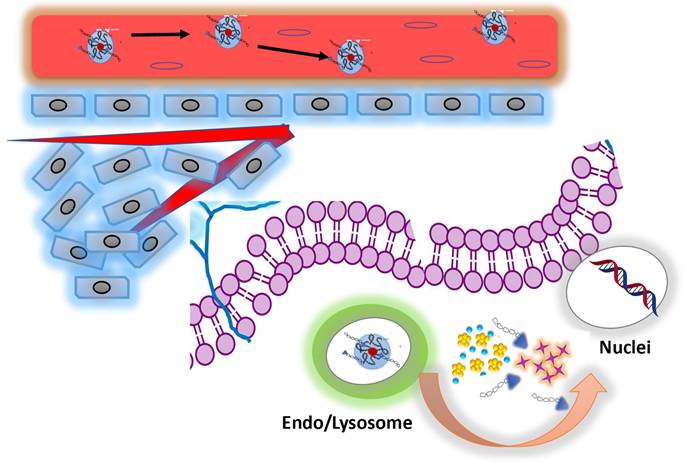
To evaluate a synergistic action of our nanoformulation, we assume that the combination of DOX and BIOT-NFL, influence the therapeutic effect and biological cellular response (Scheme 2). On the basis of our previous studies27,36, we hypothesized that DOX IN-NFL@AuNPs was stable and could penetrate the cell membrane via lysosomal-mediated pathway61.
We think that BIOT-NFL was released, interacting with receptors and inhibiting angiogenesis and tumorigenesis through destroying of microtubules (Scheme 2). There is a synergistic effect between DOX and the peptide. Thus, the peptide weakens the cytoskeleton of cells which could improve the effect of DOX. In a related way, DOX released under gold complex, acts on nuclei inhibiting DNA mutated replication40. This behavior is very encouraging in order to promote the synergic effect of peptides and chemotherapeutic drugs increasing the immune responses.
Histological sections of the heart, pancreas and tumor stained by H&E.
Schematic representation for mechanism of co-delivery of doxorubicin and BIOT-NFL by complexation method for enhanced synergistic cancer therapy.
Conclusions
This study reports the potential of DOX IN-NFL@AuNPs to target pancreatic cancer and to improve the synergic anti-tumor efficacy. On the basis of these findings, DOX IN-NFL@AuNPs displayed a significant repression of tumor growth, and higher stimulation of the immune system. Both the data and the new conception of those peptides, present a major synchronized driver to implement the numerical output data provided by the hypothesis computational model, that define the dynamic of the micro-environment of ECM and the multi-physics fluid conditions. Thus, the DOX IN-NFL@AuNPs is promoted to contribute to divert the therapy resistance out of their field of interaction with PDAC, hence, compromising the pro-tumorigenic programs in early stage, or isolate the tumor microenvironment center (TMC) within a sever electromechanical environment. We confirm that the chemical-molecular conception and design plays a key role in the parameters modulating the in vivo properties and functionalities of the nano carriers, improving their performance in cancer therapy.
Materials and Methods
Tetrachloroauric acid (HAuCl4*H2O), sodium borohydride (NaBH4), dicarboxylic PolyEthylene Glycol (PEG)-600 (PEG) (PEG-diacide), phosphate buffered saline (PBS, 0.1 M, pH from 4 to 13), DMEM, Doxorubicin (DOX) (98%), sodium chloride NaCl (0.9%; 99.5%) EDTA, Isoflurane, Paraformaldehyde were purchased by Sigma-Aldrich at maximum purity grade. All solvents were used without any further purification. BIOT-NFL-peptide, was produced by Polypeptide Group (Strasbourg, France). Experiments were carried out at room temperature if not specified otherwise.
Synthesis of DOX IN-PEG-AuNPs
The synthesis of DOX IN-PEG-AuNPs colloids, used in this study as control, was described previously21. Briefly 20 mL of aqueous HAuCl4 ( 0.8 mM ) was mixed with 2 mL of DOX at 1 mg/ mL for 1h under stirring at room temperature. After 10 min, 1 mL of PEG diacide at 1mg/mL was added to the solution. Finally, 3 mL of ice-cold 7,93 mM NaBH4 was added dropwise.
Synthesis of BIOT-NFL-PEG-AuNPs
The synthesis of BIOT-NFL-PEG-AuNPs colloids, used in this study as control, was described previously27. Briefly 20 mL of HAuCl4 aqueous dispersion (0.8 mM) was added to the NFL-peptide solution (0.05 mL, 1 mg mL 1 in water/10% ethanol) and stirred for 20 minutes. Then, 250 mL of PEG diacide (1mM) was added and mixed by magnetic stirring at room temperature. Finally, 1.8 mL of NaBH4 (3 mg/10 mL) was added at once. The formation of the BIOT-NFL-PEG-AuNPs was indicated by an instantaneous color change of the dispersion from pale yellow to bright pink-purple after the addition of the reducing agent. The “as-prepared” BIOT-NFL-PEG-AuNP dispersion was purified by centrifugation three times at 9 000 rpm for 9 minutes; then, the supernatant was discarded.
Synthesis of DOX IN-NFL@AuNPs
20 ml HAuCl4 aqueous solution (2.5×10-4 M) was added to 100µl of NFL-DOX solution and was stirred for 30 min. After 30 min, 250 µl of dicarboxylic PEG was added and mixed by magnetic stirring for 10 min at room temperature. Finally, 1.2 ml of aqueous 0.01 M NaBH4 was added at once. The formation of the DOX IN-NFL@AuNPs was confirmed by a color change of the solution after reduction (NaBH4) and proved by UV-VIS and Raman Spectroscopy (data not show).
Products of each synthetic step were stored at 27-29°C and characterized by UV-Vis spectroscopy and Transmission Electron Microscopy (TEM). The “as-prepared” DOX IN-NFL@AuNPs solution was centrifuged at 6,000 rpm for 10 min for three times; then, the supernatant was discarded. This was repeated twice to remove excess of not-conjugated dicarboxylic PEG.
Preparation of DOX-NFL (DOX-NFL)
The BIOT-NFL in powder was diluted in water and ethanol (100/900µl) at 1.8 nM of concentration. A DOX solution was prepared at 17µM of concentration. 200 µl de BIOT NFL was then diluted to 980 µl of DOX.
Physicochemical evaluation
All the measurements were performed in triplicate in order to validate the reproducibility of the synthetic and analytical procedures. All the measurements were carried out as previously described21.
UV/Vis measurements
The absorption spectra of all NPs were recorded in water at a concentration of 10-4 M. All spectra were recorded using a Perkin Elmer Lambda UV/Vis 950 spectrophotometer in plastic cuvettes with an optical path of 10 mm. The wavelength range was 200-900 nm.
Transmission Electron Microscopy (TEM)
All microscopy analyses were realized as previously described21. Briefly 2 mL of each sample was deposited on copper grids (150 mesh) and stained with 2% uranyl acetate for one minute, and then each sample was dried at room temperature before observation. The examination was performed usinga120 kV Jeol JEM-1400 electron microscope (Jeol, Japan) equipped with a Gatan SC1000 ORIUS® CCD camera (11 Megapixel) from the USA.
Dynamic light scattering (DLS)
The size measurements were performed using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) equipped with a He-Ne laser (633 nm, fixed scattering angle of 173°) at room temperature.
Zeta potential measurements
All measurements were carried out as previously described21.
Loading and release
All experiments about drug/peptide loading and release were carried out as previously described19,21.
Supplementary Material
Supplementary methods, figures and tables.
Acknowledgements
We thank ERGANEO-SATT for this study. This work has been partly performed on the CNanoMat platform of the University Paris 13 and Guangzhou University of Traditional Chinese Medicine" as "delivery of service".
This work was funded by the National Natural Science Foundation of China (82273291) from Xiaowu Li, Shenzhen Natural Science Fund (the Stable Support Plan Program [No.20220810151804002]) from Hui Liu, and the Sanming Project of Medicine in Shenzhen (grant numbers SZSM202003009) from Xiaowu Li. HaiYa Young Scientist Foundation of Shenzhen University General Hospital (HY008) from Hui Liu.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ushio J, Kanno A, Ikeda B. et al. Pancreatic Ductal Adenocarcinoma: Epidemiology and Risk Factors. Diagnostics. 2021 11
2. Lan X, Robin G, Kasnik J. et al. Challenges in Diagnosis and Treatment of Pancreatic Exocrine Insufficiency among Patients with Pancreatic Ductal Adenocarcinoma. Cancers. 2023 15
3. Quiñonero F, Mesas C, Doello K. et al. The challenge of drug resistance in pancreatic ductal adenocarcinoma: a current overview. Cancer Biology and Medicine. 2019;16:688-699
4. Schober M, Jesenofsky R, Faissner R. et al. Desmoplasia and chemoresistance in pancreatic cancer. Cancers. 2014;6:2137-54
5. Chandana S, Babiker HM, Mahadevan D. Therapeutic trends in pancreatic ductal adenocarcinoma (PDAC). Expert Opin Investig Drugs. 2019;28:161-177
6. Wu F, Yang J, Liu J. et al. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6:021-00641
7. Apte M. V, Yang L, Phillips P. A, et al. Extracellular matrix composition significantly influences pancreatic stellate cell gene expression pattern: role of transgelin in PSC function. Am J Physiol Gastrointest Liver Physiol. 2013;305:6 18
8. Rasheed ZA, Matsui W, Maitra A. Pathology of pancreatic stroma in PDAC
9. Wang L. M, Silva, M. A, D'Costa, Z,et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:4183-94
10. Hosein A. N.; Brekken, R. A.; Maitra, A, Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17(8):487-505
11. Bent E. H.; Millán-Barea, L. R.; Zhuang, I.; et al. Microenvironmental IL-6 inhibits anti-cancer immune responses generated by cytotoxic chemotherapy. Nat Commun. 2021;12(1):021-26407
12. Haynes N. M.; van der Most, R. G.; Lake, R. A. et al Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20:545-57
13. Najibi A. J.; Larkin, K.; Feng, Z.; et al Chemotherapy Dose Shapes the Expression of Immune-Interacting Markers on Cancer Cells. Cell Mol Bioeng. 2022;15:535-551
14. Mortara L., Balza E., Bruno A.. et al. Anti-cancer Therapies Employing IL-2 Cytokine Tumor Targeting: Contribution of Innate, Adaptive and Immunosuppressive Cells in the Anti-tumor Efficacy. Front Immunol. 2018;9:2905
15. Jiang M, Zeng J, Zhao L. et al. Chemotherapeutic drug-induced immunogenic cell death for nanomedicine-based cancer chemo-immunotherapy. Nanoscale. 2021;13(41):17218-17235
16. Li H, Feng Y, Luo Q. et al. Stimuli-activatable nanomedicine meets cancer theranostics. Theranostics. 2023;13(15):5386-5417
17. Patra J.K, Das G, Fraceto LF. et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018:16-7
18. Palmerston Mendes L, Pan J, Torchilin VP. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules. 2017;23229:1401
19. An H, Deng X, Wang F, Xu P. et al. Dendrimers as Nanocarriers for the Delivery of Drugs Obtained from Natural Products. Polymers (Basel). 2023 121510:2292
20. Xing H, Hwang K, Lu Y. Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics. 2016;69:1336-52
21. Chandrakala V, Aruna V. & Angajala, G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. emergent mater. 2002;5:1593-1615 (
22. Sun H, Meng F, Cheng R. et al. Reduction-responsive polymeric micelles and vesicles for triggered intracellular drug release. Antioxid Redox Signal. 2014;215:755-67
23. Felber AE. et al. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Advanced Drug Delivery Reviews. 2012;11:64
24. Duncan B. et al. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. Journal of Controlled Release. 2010;148:1
25. Mikhailova EO. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J Funct Biomater. 2021;23:70
26. Banstola A, Emami F, Jeong J.-H. et al. Current Applications of Gold Nanoparticles for Medical Imaging and as Treatment Agents for Managing Pancreatic Cancer. Macromolecular Research. 2018;26:955-964
27. Liu Q, Liu H, Griveau A. et al. NFL-TBS.40-63 Peptide Gold Complex Nanovector: A Novel Therapeutic Approach to Increase Anticancer Activity by Breakdown of Microtubules in Pancreatic Adenocarcinoma (PDAC). ACS Pharmacology & Translational Science. 2022;5:1267-1278
28. Liu Q, Liu H, Sacco P. et al. CTL-doxorubicin (DOX)-gold complex nanoparticles (DOX-AuGCs): from synthesis to enhancement of therapeutic effect on liver cancer model. Nanoscale Adv. 2020;2:5231-5241
29. Liu Q, Sacc P, Marsich E. et al. Lactose-Modified Chitosan Gold (III)-PEGylated Complex-Bioconjugates: From Synthesis to Interaction with Targeted Galectin-1 Protein. Bioconjug Chem. 2018;29:3352-3361
30. Moustaoui H, Movia D, Dupont N. et al. Tunable Design of Gold (III)-Doxorubicin Complex-PEGylated Nanocarrier. The Golden Doxorubicin for Oncological Applications. ACS Applied Materials & Interfaces. 2016;8:31 19946-19957
31. Karim R, Lepeltier E, Esnault L. Enhanced and preferential internalization of lipid nanocapsules into human glioblastoma cells: effect of a surface-functionalizing NFL peptide. Nanoscale. 2018;10:13485-13501
32. Mellinger A, Lubitz L. J, Gazelle C, the use of liposomes functionalized with the NFL-TBS.40-63 peptide as a targeting agent to cross the in vitro blood-brain barrier and target glioblastoma cells. International Journal of Pharmaceutics. 2023;646:123421
33. Alnemeh-Al Ali H, Artzner GA. Investigation on the self-assembly of the NFL-TBS.40-63 peptide and its interaction with gold nanoparticles as a delivery agent for glioblastoma. Int J Pharm X. 2022;4:100128
34. Barreau K, Montero-Menei C, Eyer J. The neurofilament derived-peptide NFL-TBS.40-63 enters in-vitro in human neural stem cells and increases their differentiation. PLoS One. 2018;13:8
35. Arib C, Liu H, Liu Q. et al. A Pegylated Flavin Adenine Dinucleotide PEG Complex to Boost Immunogenic and Therapeutic Effects in a Liver Cancer Model. Nanotheranostics. 2021;54:405-416
36. Arib C, Griveau A. et al. Cell penetrating peptide (CPP) gold(iii) - complex - bioconjugates: from chemical design to interaction with cancer cells for nanomedicine applications. Nanoscale Adv. 2022;414:3010-3022
37. Griveau A, Alnemeh-Al Ali H, Jourdain M. et al. Characterization and quantification of the interaction between the NFL-TBS.40-63 peptide and lipid nanocapsules. Int J Pharm X. 2022;4:100127
38. Wordeman L, Vicente JJ, Microtubule Targeting Agents in Disease. Classic Drugs, Novel Roles. Cancers. 2021;13:22
39. Fan D, Cao Y, Cao M. et al. Nanomedicine in cancer therapy. Signal Transduction and Targeted Therapy. 2023;8:293
40. Kciuk M, Gielecińska A, Mujwar SR. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells. 2023;12:4
41. Christowitz C, Davis T, Isaacs A. et al. Mechanisms of doxorubicin-induced drug resistance and drug resistant tumour growth in a murine breast tumour model. BMC Cancer. 2019;19:757
42. Cox J, Weinman S. Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepat Oncol. 2016;3:57-59
43. Arachchige M. P. Laha S. S, Naik A, et al. Functionalized nanoparticles enable tracking the rapid entry and release of doxorubicin in human pancreatic cancer cells. Micron. 2017;92:25-31
44. Gurunathan S, Kang M. H, Qasim M, et al. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int J Mol Sci. 2018;19:10
45. Khan M, Boumati S, Arib C. et al. Doxorubicin (DOX) Gadolinium-Gold-Complex: A New Way to Tune Hybrid Nanorods as Theranostic Agent. Int J Nanomedicine. 2021;16:2219-2236
46. Khan M, Liu H, Sacco P. et al. DOTAREM (DOTA)-Gold-Nanoparticles: Design, Spectroscopic Evaluation to Build Hybrid Contrast Agents to Applications in Nanomedecine. Int J Nanomedicine. 2022;17:4105-4118
47. Asensio-López M. C, Sole F, Pascual-Figa D. Doxorubicin-induced oxidative stress: The protective effect of nicorandil on HL-1 cardiomyocytes. PLoS One. 2017;12:2
48. Berges R, Balzeau J, Peterson AC. et al. A tubulin binding peptide targets glioma cells disrupting their microtubules, blocking migration, and inducing apoptosis. Mol Ther. 2012;20:1367-77
49. Nekrasov O. E, Mendez M. G, Chernoivanenko I. S, et al. Vimentin intermediate filaments modulate the motility of mitochondria. Mol Biol Cell. 2011;22:2282-9
50. Zhao H, Wu L, Yan G. et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduction and Targeted Therapy. 2021;6:263
51. Russo M. et al. Metabolic Aspects of Anthracycline Cardiotoxicity. Curr Treat Options Oncol. 2021;22:020-00812
52. Pop V.-V, Seicean A, Lupan I. et al. IL-6 roles - Molecular pathway and clinical implication in pancreatic cancer - A systemic review. Immunology Letters. 2017;181:45-50
53. Wang M, Zhai X, Li J. et al. The Role of Cytokines in Predicting the Response and Adverse Events Related to Immune Checkpoint Inhibitors. Front Immunol. 2021;12:670391
54. Jang D. I, Lee A. H, Shin H. Y, et al The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021;22:5
55. Josephs S. F, Ichim T. E, Prince, S. M,et al. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. Journal of Translational Medicine. 2018;16:242
56. Tanaka T, Narazaki M, Kishimoto T. et al. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:10
57. Kim M, Jung K, Kim I. et al. TNF-α induces human neural progenitor cell survival after oxygen-glucose deprivation by activating the NF-κB pathway. Experimental & Molecular Medicine. 2018;50:1-14
58. Jorgovanovic D, Song M, Wang L. et al. Roles of IFN-γ in tumor progression and regression: a review. Biomark Res. 2020;8:020-00228
59. Xiong Y, Gao W, Xia F. et al. Peptide-Gold Nanoparticle Hybrids as Promising Anti-Inflammatory Nanotherapeutics for Acute Lung Injury: In Vivo Efficacy, Biodistribution, and Clearance. Adv Healthc Mater. 2018;7:19 12
60. Abdullah CS, Alam S, Aishwarya R. et al. Doxorubicin-induced cardiomyopathy associated with inhibition of autophagic degradation process and defects in mitochondrial respiration. Scientific Reports. 2019;9:2002
61. Seebacher NA, Richardson DR Jansson PJ. A mechanism for overcoming P-glycoprotein-mediated drug resistance: novel combination therapy that releases stored doxorubicin from lysosomes via lysosomal permeabilization using Dp44mT or DpC. Cell Death & Disease. 2016;7:2510-2510
62. Chattopadhyay S. et al. Synthetic Immunogenic Cell Death Mediated by Intracellular Delivery of STING Agonist Nanoshells Enhances Anticancer Chemo-immunotherapy. Nano Lett. 2020;20(4):2246-2256
Author contact
* Corresponding author: Jolanda Spadavecchia (jolanda.spadavecchiafr; jolanda.spadavecchiacom).

 Global reach, higher impact
Global reach, higher impact