ISSN: 2206-7418
Nanotheranostics 2020; 4(2):57-70. doi:10.7150/ntno.39804 This issue Cite
Research Paper
Effects of molecular weight and structural conformation of multivalent-based elastin-like polypeptides on tumor accumulation and tissue biodistribution
1. Department of Biochemistry and Cell Biology, School of Medicine, and Cell & Matrix Research Institute, Kyungpook National University, Daegu 41944, Republic of Korea.
2. Department of Pathology, All India Institute of Medical Sciences, New Delhi-110029, India.
3. Department of Medical Oncology Lab., All India Institute of Medical Sciences, New Delhi-110029, India.
4. Laboratory Animal Center, Daegu-Gyeongbuk Medical Innovation Foundation, Cheombok, Daegu, 41061, Republic of Korea
*Vijaya Sarangthem, Bo-Yeon Seo and Aena Yi equally contributed to this work
Abstract
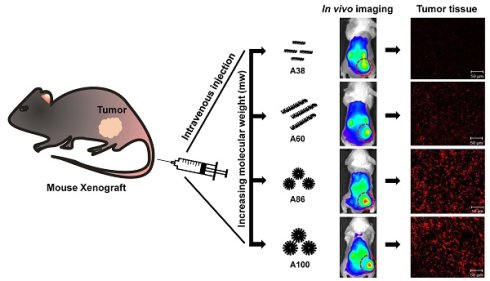
In order to improve clinical outcomes for novel drug delivery systems, distinct optimization of size, shape, multifunctionality, and site-specificity are of utmost importance. In this study, we designed various multivalent elastin-like polypeptide (ELP)-based tumor-targeting polymers in which multiple copies of IL-4 receptor (IL-4R)-targeting ligand (AP1 peptide) were periodically incorporated into the ELP polymer backbone to enhance the affinity and avidity towards tumor cells expressing high levels of IL-4R. Several ELPs with different molecular sizes and structures ranging from unimer to micelle-forming polymers were evaluated for their tumor accumulation as well as in vivo bio-distribution patterns. Different percentages of cell binding and uptake were detected corresponding to polymer size, number of targeting peptides, or unimer versus micelle structure. As compared to low molecular weight polypeptides, high molecular weight AP1-ELP showed superior binding activity with faster entry and efficient processing in the IL-4R-dependent endocytic pathway. In addition, in vivo studies revealed that the high molecular weight micelle-forming AP1-ELPs (A86 and A100) displayed better tumor penetration and extensive retention in tumor tissue along with reduced non-specific accumulation in vital organs, when compared to low molecular weight non-micelle forming AP1-ELPs. It is suggested that the superior binding activities shown by A86 and A100 may depend on the multiple presentation of ligands upon transition to a micelle-like structure rather than a larger molecular weight. Thus, this study has significance in elucidating the different patterns underlying unimer and micelle-forming ELP-mediated tumor targeting as well as the in vivo biodistribution.
Keywords: multivalent, size-dependent, tumor targeting, IL-4 receptor, ELP, biodistribution

 Global reach, higher impact
Global reach, higher impact