ISSN: 2206-7418
Nanotheranostics 2025; 9(3):289-298. doi:10.7150/ntno.120358 This issue Cite
Research Paper
Novel Phase Shift Microbubbles, MVT-101, Enhance Sonothrombolysis in a Porcine Model of Deep Vein Thrombosis
1. Microvascular Therapeutics Inc, 1635 E. 18 th Street, Tucson AZ 85719, USA.
2. University of North Carolina, 109 Mason Farm Road, Chapel Hill, NC 27599, USA.
3. Synchrony Labs, 3908 Patriot Drive, Suite 170, Durham, NC 27703, USA.
4. University of Arizona Cancer Center, 1515 N. Campbell Ave, Tucson AZ 85724, USA.
Received 2025-6-26; Accepted 2025-10-14; Published 2025-10-24
Abstract
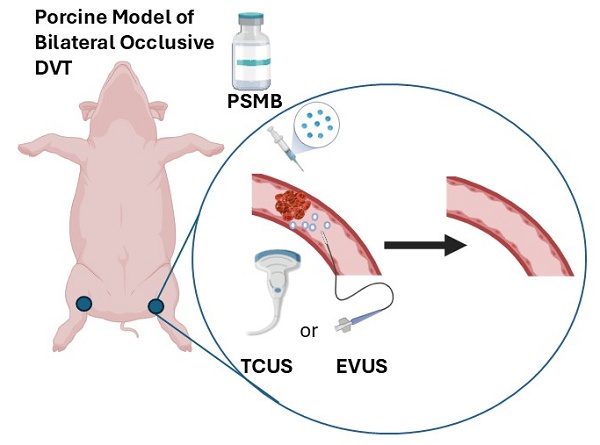
Background: Estimates suggest that 60,000-100,000 Americans die of Deep Vein Thrombosis / Pulmonary Embolism (DVT/PE) each year. Between 10 to 30% of people with PE will die within one month of diagnosis. Sudden death is the first symptom in about 25% of people who have PE. Among people who have had DVT, one third to one half will have long-term complications (post-thrombotic syndrome) such as swelling, pain, discoloration, and scaling in the affected limb. Microbubble (MB) enhanced sonothrombolysis (SL) has shown promise in treating vascular thrombosis, but MBs are micrometer-sized structures and do not easily permeate clots. We hypothesized that sub-micron structures, e.g., phase shift microbubbles (PSMB or nanodroplets, herein referred as MVT-101) would better permeate clots for more effective SL. We have already shown that MVT-101 (~250 nm in diameter size), easily penetrate the thrombus and demonstrate increased SL efficiency in the rat hindlimb model of microvascular obstruction (MVO).
Methods: Bi-lateral occlusive model of DVT was created in the pigs. Endovascular ultrasound was delivered using the EKOS™ Endovascular System and transcutaneous ultrasound (TCUS) was delivered using a diagnostic transducer (GE Vivid E9).
Results: We now report that in combination with the EKOS™ Endovascular System, MVT-101 made with perfluoropropane, dissolved occlusive DVT clots in a bi-lateral occlusive porcine model of DVT in combination with (Mean clot resolution = 45.12 ± 46.79 %) or without tissue Plasminogen Activator (tPA) (Mean clot resolution = 36.00 ± 52.27 %) within 60 min of treatment time. We also show that TCUS further SL was observed in the presence of MVT-101 and tPA (Mean clot resolution 76.21 ± 11.86 % with 1000-2000 mg tPA) as compared to tPA alone (Mean clot resolution 16.75 ± 17.00 % with 1000 mg tPA).
Conclusion: Both procedures proved to be safe and did not produce PE. In conclusion, MVT-101 holds great potential as therapeutic agent for treating DVT either with TCUS or endovascular ultrasound (EVUS).
Keywords: sonothrombolysis, phase shift microbubbles, porcine model, deep vein thrombosis
Introduction
Deep Vein Thrombosis (DVT) is a serious disease which affects over 600,000 patients a year in the USA. About 40% of acute DVT develop following major surgery such as cancer or hip/knee replacements. Estimates suggest that 60,000-100,000 Americans die of DVT/Pulmonary Embolism (PE) (venous thromboembolism) each year [1]. Between 25% of people with PE will die within one month of diagnosis [1]. By far, the major cause of PE is propagation or embolization of clot from DVT into the pulmonary arteries [2]. Among people who have had DVT, one third to one half will have long-term complications (post-thrombotic syndrome) such as swelling, pain, discoloration, and scaling in the affected limb [1]. While the current standard of care for DVT includes anti-coagulation for mild cases and tissue plasminogen activator (tPA) with endovascular catheter ultrasound for more severe cases [3], most post-operative patients cannot tolerate anti-coagulation or tPA because of the risk of bleeding. There is a clear unmet medical need to develop safer and rapid treatment for acute DVT.
Despite the use of anticoagulant therapy, post-thrombotic syndrome (PTS) develops within 2 years in approximately half of patients with proximal DVT [4-7]. PTS commonly causes chronic limb pain and swelling and can progress to cause major disability, leg ulcers, and impaired quality of life [8, 9]. Pharmacomechanical catheter-directed thrombolysis (PCDT) delivers a fibrinolytic drug into the thrombus with concomitant thrombus aspiration/agitation [10]. The objective of pharmaco-mechanical thrombolysis is to remove thrombus using low-dose fibrinolysis and mechanical agitation, to reduce the risk of PTS while minimizing the risk of bleeding [11-14]. The Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) trial demonstrated that the addition of PCDT to anticoagulation did not result in a lower risk of the PTS but did result in a higher risk of major bleeding [15]. More recently, ultrasound has also been deployed as an effective technique for PCDT therapy [16, 17]. The EKOS™ endovascular ultrasound catheter is FDA approved and uses targeted ultrasonic waves in combination with clot-dissolving drugs in the treatment of pulmonary emboli (PE) [18]. Ultrasonic waves delivered from the catheter improve the permeation of tPA into the clot. The thrombolytic dose can be reduced by up to 88-92% with the EKOS catheter as compared to the standard systemic treatment [19, 20].
Further, numerous reports have shown that microbubble contrast agents (MB) enhance the rate of sonolysis with ultrasound [21-24]. In vitro MB-enhanced sonolysis studies have also shown that clot lysis with this technique does not produce large fragments but rather dissolves clot into constituent components of fibrin, platelets and red blood cells [25]. In one in vitro study, evaluating MB-enhanced sonolysis with low frequency ultrasound (20 kHz), the residual median microparticle size was 320 nm for ultrasound and MB (range 197-462 nm; p > 0.05) [25]. Additionally, in a study examining forward looking transducers for microbubble-mediated sonothrombolysis, a mean thrombolysis rate of 1.4 ± 0.33 mg/min was achieved without the use of a thrombolytic agent [26]. In a model of thrombus in the superior vena cava in pigs, MB enhanced sonothrombolysis was successful in dissolving the thrombus and pulmonary scintigraphy showed no evidence of PE [27].
Subsequently, the Microbubbles (MB) and UltraSound accelerated Thrombolysis trial (MUST Trial) for peripheral arterial occlusions was performed to test the efficacy and safety of MB enhanced sonolysis in peripheral arterial occlusions [28, 29]. In this trial one vial of Luminity was infused every 15 min while patients were treated for 60 min [28, 29]. MB were infused through a McNamara catheter placed intra-arterially in the distal segment of the thrombus [28, 29]. The MUST Trial demonstrated that the treatment was feasible and provided evidence of safety without side effects related to the MB, as well as significant improvement in both arterial flow and pain scores [29].
Thrombi have variably porous structures composed of fibrin, platelets and red blood cells [30]. Phase Shift Microbubbles (PSMB) are smaller diameter than MB (e.g. 150-350 nm versus 1-3 microns for MB) [23]. Our PSMB (herein referred to as MVT-101) are made of octafluoropropane gas encapsulated into a lipid shell made of phospholipids such as dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylethanolamine (DPPE), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N- [methoxy(polyethylene glycol)-5000] (ammonium salt) (DPPE-MPEG-5000), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- methoxy(polyethylene glycol)-5000 (DSPE-MPEG-5000) as described in previous studies [31]. As the clot progresses from an acute clot with relatively large pores, to the sub-acute or chronic stages where fibrin networks become denser with smaller pores, clot permeability decreases, limiting the penetration of MB [32]. Because of their smaller diameter, PSMB better permeate thrombi than MB to afford more effective sonolysis [23, 33-35]. An in vitro study showed that PSMB enabled sonolysis of retracted, hard clots, which has generally not been achievable with MB sonolysis [36-38]. In this study, we tested for the first time both endovascular US (EKOS Endovascular System) and transcutaneous US (GE Vivid E9) in combination with MVT-101 for sonothrombolysis in a porcine model of DVT.
Material and Methods
Formulation of MVT-101
For protocols detailing the manufacture and preparation of MVT-101, please refer to previously published methods [31]. MVT-101 was first activated and condensed prior to administration. For endovascular treatments 2.6 mL of MVT-101 in 20 mL of saline was administered over 1 hour. For transcutaneous treatments 5.2 mL of MVT-101 in 20 mL of saline was administered over 1 hour. The PSMBs used in this study had an average size of approximately 250-350 nm and as previously described [31].
DVT porcine model
The animal studies were performed at Synchrony Labs (Durham, North Carolina) under an approved protocol (IACUC 313-01-21). Yorkshire pigs (male and female, ~70kg) were anesthetized with isoflurane gas and ventilated on 100% O2 during the procedure. The animals were monitored for blood pressure, heart rate, EKG and pulse oximetry during the procedure. Bilateral iliac vein occlusions were created by inflating a balloon catheter to occlude flow and injecting thrombin, following a protocol adapted from [39]. X-ray fluoroscopy was used to introduce balloon catheterization, and venography was performed to visualize the venous anatomy. The clots were allowed to mature for at least 90 minutes and up to 4 hours prior to treatment. Angiography was performed to confirm clot position, size, and degree of occlusion.
Transcutaneous ultrasound and MVT-101 treatment
For the transcutaneous ultrasound (TCUS) treatment, 4 vials of MVT-101 were infused (administered in a 20 mL volume at a rate of 20 mL/hr) for a total treatment time of 60 minutes. MVT-101 were infused in 6 animals using the multi-side hole Cragg McNamara catheter positioned alongside or within the clot with doses of tPA ranging from 0 to 2,000 micrograms. A GE Vivid E9 system using the 4V-D probe was used for imaging and for sonolysis for the TCUS experiments. Initial imaging was performed with a linear array 9L probe for localization and measurement of flow on Doppler. A Vivid E9 4V-D probe was used for sonolysis. The probe was operated in harmonic mode with a transmit frequency of 1.5 MHz and MI = 1.4 at a frame rate of 46.2-54.1 Hz and depth = 6-12 cm for 60 minutes during infusion of MVT-101 administered intravascularly via syringe infusion pump. The transmit frequency of 1.5 MHz was utilized for PSMB activation. For TCUS treatment, tPA was included within the 20 mL diluted solution of MVT-101. For the control group no treatment was administered.
Endovascular ultrasound
The EKOS™ catheter was advanced into the clot using 12-cm length acoustic model operating at 2-3 MHz. For the MVT-101, 2 vials were prepared in 20 cc saline and infused through the sheath while ultrasound energy was applied through the catheter. For endovascular ultrasound (EVUS) treatment, tPA was administered through the drug port of the EKOS™ system at a rate of 35 mL/hr with dosages ranging from 0 to 769 micrograms while ultrasound was applied. Various conditions such as tPA, no tPA, and no MVT-101 versus MVT-101 were tested, infusing the same volumes of saline as control.
Quantification of sonothrombolysis
The length and width of clots were measured using angiography. Post procedure, the percent resolution of the clot was estimated visually by one reviewer. The volume of the clot was also calculated by using the formula for volume of a cylinder, π x length x radius2 and the % resolution was obtained using the formula: ((π x length x radius2 pre) - (π x length x radius2 post treatment))/ (π x length x radius2 pretreatment) x 100. The clots were measured onsite post treatment (first blinded reviewer), and a second reviewer (a blinded radiologist) reviewed and measured the clots offsite. The final measurements used for analysis were the data averaged of both onsite and offsite measurements obtained from the two reviewers.
Whole blood chemistry and hematology analysis
Whole blood chemistry and hematology analysis was performed by Quality Veterinary Laboratory (Davis, CA).
Statistical analysis
Throughout the manuscript, data were expressed as mean ± standard deviation. For the transcutaneous data, normality and homogeneity of variance were confirmed using the Shapiro-Wilk test and F-tests. One-way ANOVA was then performed to assess differences between groups, followed by Tukey's test to identify individual group differences. For the endovascular data, Shapiro-Wilk and F-tests indicated homogeneity of variance but a non-normal distribution. Therefore, the Kruskal-Wallis test was employed to evaluate significant differences between groups.
Results
Sonothrombolysis using transcutaneous ultrasound
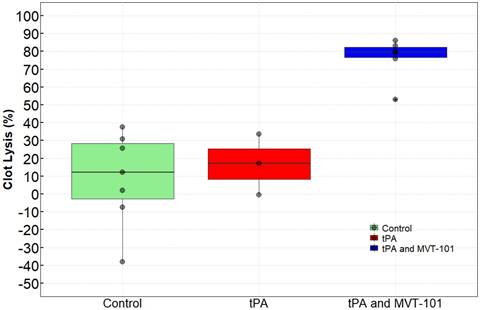
Pigs that had bilateral occlusive iliac DVT received four doses of MVT-101 that were infused (with 2 doses per 10 mL dose administered at a rate of 20 mL/hr)) for a total treatment time of 60 minutes. There was no significant change in blood pressure, heart rate, or oxygen saturation during the treatment, as shown by the parameters measured and summarized in Table 1. Table 2 details the size the percent reduction in clot size (% resolved) in each pig and Figure 1 represents the averaged results for each treatment group. Of note, TCUS SL effects were observed as early as 20 min post-treatment with MVT-101+tPA (data not shown). While only 8.97±26.17% was calculated with no tPA and no PSMB; the clot resolution increased to 16.75±17.00% in the presence of 1,000 mg tPA and 76.21 ±11.86 with 1,000 mg-2000 mg tPA in combination with MVT-101. The ANOVA test revealed a difference between the groups and a Tukey test revealed that the tPA and MVT-101 group was significantly different from both the tPA condition and the control condition (p=0.0001 as compared to control; p=0.0032 as compared to tPA alone). There was no significant difference between the tPA condition and the control condition (p=0.8467). Blood samples were analyzed for general blood chemistry and comprehensive metabolic panel (Tables 3 and 4) pre- and post-treatment with TCUS, MVT-101 and tPA. Whole blood chemistry and most of the whole blood hematology analyses did not reveal any significant changes between baseline and post-treatment. The sole indicator in the whole blood hematology analysis that changed significantly was the Neutrophil-to-Lymphocytes ratio (NLR), with NLR of ~0.47 pre-treatment and NLR of ~2.97 post-treatment. The increase in this ratio was likely caused by acute DVT [40] since the blood samples were taken right after the treatment (Table 3). We noted also a decrease in albumin (ALB) and total protein (TP) post-treatment as compared to baseline. Fibrinogen was also shown to be unchanged (152±22 ng/ml versus 153±23 ng/ml pre- and post-treatment respectively) indicating no hemorrhagic condition.
Parameters at baseline and post-treatment with transcutaneous ultrasound, MVT-101 and tPA. Data are the mean ± standard deviations, number of pigs, n =5, and * for p<0.05; ** for p<0.01 and *** for p<0.005.
| Parameters | Baseline | End of Study | p value |
|---|---|---|---|
| Pulmonary Artery Pressure (PAP) | 20.0±4.3 | 18.8±6.2 | 0.72 |
| Pulmonary Capillary Wedge pressure (PCW) | 11.2±3.4 | 9.8±3.0 | 0.51 |
| Oxygen Saturation (sO2) | 99.3±1.5 | 99.3±1.5 | 0.82 |
| Heart Rate (Pulse Oximetry) | 85.3±9.8 | 91.5±17.5 | 0.55 |
| Mean arterial pressure (MAP) | 70.0±8.5 | 66.3±7.4 | 0.53 |
| Systolic pressure (SYS)*** | 89.0±16.2 | 53.8±7.6 | 0.002 |
| Diastolic pressure (DIA)** | 74.6±6.9 | 55.2±9.4 | 0.006 |
Transcutaneous ultrasound dataset. Dataset obtained for each pig. L, for left; R, for right; tPA for tissue plasminogen Activator. NA, for not available.
| Pig | Vein | Resolved (%) | Treatment | Clot Mature Time (minutes) | tPA (µg) |
|---|---|---|---|---|---|
| 8 | L iliac vein | 80.11 | tPA with MVT-101 | 102 | 2000 |
| 10 | L iliac vein | 83.06 | tPA with MVT-101 | 106 | 2000 |
| 11 | L iliac vein | 79.03 | tPA with MVT-101 | 111 | 2000 |
| 9 | R iliac vein | 53.06 | tPA with MVT-101 | 112 | 2000 |
| 12 | R iliac vein | 86.05 | tPA with MVT-101 | 107 | 1000 |
| 13 | L iliac vein | 75.96 | tPA with MVT-101 | 108 | 1000 |
| 8 | R iliac vein | -37.98 | Control | NA | 0 |
| 10 | R iliac vein | 1.95 | Control | NA | 0 |
| 13 | R iliac vein | 25.73 | Control | NA | 0 |
| 12 | L iliac vein | -7.50 | Control | NA | 0 |
| 11 | R iliac vein | 37.48 | Control | NA | 0 |
| 9 | L iliac vein | 30.88 | Control | NA | 0 |
| 14 | R iliac vein | 12.26 | Control | NA | 0 |
| 29 | R iliac vein | 17.13 | tPA | 110 | 1000 |
| 29 | L iliac vein | 33.56 | tPA | 215 | 1000 |
| 30 | R iliac vein | -0.44 | tPA | 133 | 1000 |
Whole blood hematology analysis at baseline and post-treatment with transcutaneous ultrasound, MVT-101 and tPA. Data are the means ± standard deviations, n=5 pigs, * for p<0.05; ** for p<0.01 and *** for p<0.005. NA, for not available.
| Test | Units | Baseline | Post-treatment | p value |
|---|---|---|---|---|
| WBC | x10³ /uL | 17.5±1.8 | 18.4±3.9 | 0.99 |
| RBC | x106/uL | 5.5±0.9 | 5.1±0.4 | 0.59 |
| HGB | g /dL | 10.2±1.5 | 9.4±0.6 | 0.51 |
| HCT | % | 31.4±4.5 | 28.8±2.1 | 0.47 |
| MCV | fL | 57.5±1.6 | 57.1±1.7 | 0.78 |
| MCH | pg | 18.7±0.6 | 18.7±0.8 | 0.97 |
| MCHC | g / dL | 32.6±0.2 | 32.8±0.6 | 0.72 |
| RDW | % | 14.8±0.6 | 14.7±0.6 | 0.88 |
| PLT | x10³ /uL | 213.8±49.5 | 197.8±54.5 | 0.85 |
| MPV | fL | 9.4±0.8 | 8.9±0.9 | 0.37 |
| %NEUT | % | 29.8±10.2 | 72.6±8.3 | 0.002, ** |
| %LYMPHS | % | 63.8±9.6 | 24.4±8.0 | 0.002, ** |
| %MONO | % | 2.0±0.9 | 0.4±0.5 | 0.01, ** |
| %EOS | % | 3.8±1.1 | 2.4±1.1 | 0.31 |
| %BASO | % | 0.4±0.5 | 0.2±0.4 | 0.35 |
| %BANDS | % | 0 | 0 | NA |
| #NEUT | x10³ /uL | 5.3±2.2 | 13.5±3.8 | 0.02, * |
| #LYMPHS | x10³ /uL | 11.1±1.8 | 4.3±1.1 | 0.002, ** |
| #MONO | x10³ /uL | 0.3±0.1 | 0.1±0.1 | 0.01, * |
| #EOS | x10³ /uL | 0.6±0.2 | 0.5±0.3 | 0.47 |
| #BASO | x10³ /uL | 0.1±0.1 | 0 | 0.35 |
| #BANDS | x10³ /uL | 0 | 0 | 0 |
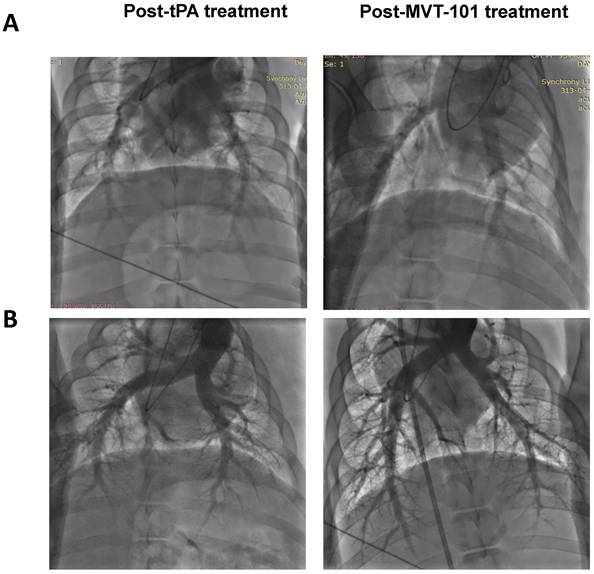
We measured several early safety parameters before and after sonothrombolysis treatment. Data are summarized in Table 1. The first set of pre-clinical safety data looked to understand and control the risk of pulmonary emboli via investigation of Pulmonary Artery Pressure (PAP), Pulmonary Capillary Wedge Pressure (PCWP), and Pulmonary Angiogram (PA). Angiograms are shown in Figure 3. All three endpoints can help ensure that pulmonary embolism did not result from the treatment of the DVT with MVT-101. The data collected for PAP, PCWP, and PA did not indicate significant differences between the baseline and end of study results, indicating the absence of pulmonary emboli during treatment with MVT-101. The second set of preclinical safety data looked at pulmonary function via Oxygen Saturation (sO2) and heart rate (using Pulse Oximetry). Again, no significant difference was seen between the baseline and end of study results, indicating maintained pulmonary function during treatment with MVT-101. The third set of preclinical safety data looked at the effect of treatment on hemodynamics via investigation of Mean Arterial Pressure (MAP), Systolic Pressure (SYS), and Diastolic Pressures (DIA). All three endpoints can help ensure that adequate blood flow was maintained. The preclinical safety data collected for MAP results did not indicate differences between the baseline and end of study results, indicating adequate blood flow was maintained.
Effects of MVT-101 and tPA in combination with transcutaneous ultrasound.
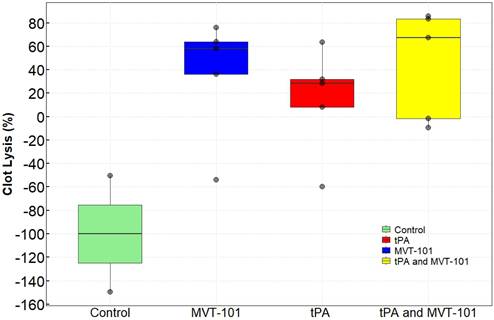
Effects of MVT-101 and Endovascular Ultrasound.
However, SYS and DIA values showed a statistically significant decrease post-treatment with TCUS, MVT-101 and tPA. Ultrasound waves can induce local vasodilation by stimulating endothelial cells to release nitric oxide, a potent vasodilator [41]. This relaxation of vascular smooth muscle in the iliac vein and surrounding vessels reduces vascular resistance, lowering both systolic and diastolic pressures. Another explanation may reside in the effective restoration of venous flow. Indeed, successful lysis of a thrombus in the iliac vein reduces obstruction, improving venous return to the heart. While this might initially increase cardiac preload, in some cases, it can lead to a transient drop in systemic vascular resistance as the previously stagnant blood re-enters circulation, potentially diluting vasoconstrictive factors and causing a temporary decrease in blood pressure, as we collected these values within 15 min. post-treatment. Of note, studies in pigs with perfluoropropane MB with same composition used to make MVT-101 (PSMB in this study), have shown that MB cleared by 20 minutes following IV administration. The mean half-life of octafluoropropane in blood after perflutren injection is 1.3 minutes, with the majority eliminated by the lungs within 10 minutes. Sheeran et al showed that perfluoropropane nanodroplets have 3.3x longer circulation time than MB [42]. Eventually, perfluoropropane in PSMB will exchange in the circulation and be exhaled by the lungs without metabolism, over a several-fold longer period of time than for perfluoropropane in microbubbles.
Sonothrombolysis using endovascular ultrasound
The data with the EKOS™ endovascular catheter are summarized in Table 5 and data averaged are shown in Figure 2. The mean resolution of the treatment with MVT-101 alone was ~36%, higher than the tPA only condition, which was ~14 %. The highest resolution was obtained with the combination of both MVT-101 and tPA with an average resolution of ~45%. Analysis by the Kruskal-Wallis test showed that there was no significant difference between the different conditions (p value of 0.1443). Of note, EVUS + MVT-101 treatment showed efficacy without tPA while the EKOS™ catheter was not effective without tPA. There was a trend to an increase in resolution of the clot when MVT-101 was utilized alone versus tPA alone. We observed a noticeable variability in all treatment conditions using EKOS™ EVUS. Overall, most of the blood compositions summarized in Tables 7 and 8 did not show major changes. As noted for the TCUS treatment, we calculated a NLR of ~0.39 pre- and of ~1.48 post-treatment. We also observed a decrease in albumin (ALB) and total protein (TP) post-treatment as compared to baseline. Parameters and angiograms (Table 6 and Figure 3B) remained unaltered during the course of the treatment with EVUS.
Pulmonary angiograms of post-tPA treatment (left) and post-MVT-101 treatment (right) with Endovascular Ultrasound (Panel A) or Transcutaneous Ultrasound (Panel B).
Whole blood chemistry analysis at baseline and post-treatment with transcutaneous ultrasound, MVT-101 and tPA. Data are the means ± standard deviations, n=5 pigs, * for p<0.05; ** for p<0.01 and *** for p<0.005.
| Test | Units | Baseline | Post-treatment | p value |
|---|---|---|---|---|
| ALB | g/dL | 2.9±0.1 | 2.5±0.1 | <0.005, *** |
| TP | g/dL | 4.9±0.2 | 4.3±0.1 | <0.005, *** |
| ALP | U/L | 97.5±33.1 | 83.8±29.2 | 0.82 |
| ALT | U/L | 24.2±6.2 | 20.0±5.2 | 0.49 |
| AST | U/L | 16.3±2.1 | 14.8±3.3 | 0.48 |
| CK | U/L | 627±254 | 640±275 | 0.85 |
| TBIL | mg/dL | 0.1±0.04 | 0.2±0.1 | 0.19 |
| DBIL | mg/dL | 0.03±0.04 | 0.1±0.1 | 0.06 |
| BUN | mg/dL | 5.3±0.9 | 6.6±1.1 | 0.13 |
| CREAT | mg/dL | 1.3±0.1 | 1.3±0.1 | 0.29 |
| CA | mg/dL | 9.3±0.2 | 9.0±0.2 | 0.03, * |
| CHOL | mg/dL | 60.3±7.8 | 52.6±9.3 | 0.29 |
| GLU | mg/dL | 91.2±33.4 | 124.2±10.8 | 0.15 |
| PHOS | mg/dL | 7.9±0.6 | 7.1±0.4 | 0.07 |
| TCO2 | mEq/L | 28.3±2.2 | 26.2±1.6 | 0.12 |
| NEUT | mEq/L | 140.7±1.8 | 136.6±1.8 | 0.01, * |
| K | mEq/L | 4.2±0.3 | 4.1±0.2 | 0.29 |
| CL | mEq/L | 102.8±1.2 | 100.0±1.2 | 0.01, * |
| GLOB | g/dL | 2.1±0.1 | 1.8±0.1 | 0.01, * |
| A/G | Ratio | 1.4±0.1 | 1.4±0.1 | 0.72 |
| B/C | Ratio | 4.2±0.7 | 5.2±0.4 | 0.05, * |
| IBIL | mg/dL | 0.08±0.07 | 0.10±0.44 | 0.61 |
| ANION | mEq/L | 13.7±1.9 | 14.4±1.5 | 0.31 |
Endovascular ultrasound dataset. Dataset obtained for each pig. L, for left; R, for right; tPA for tissue plasminogen Activator.
| Pig | Vein | Resolved (%) | Treatment | Clot Mature Time (minutes) | TPA (µg) |
|---|---|---|---|---|---|
| 15 | L iliac vein | 83.46 | tPA with MVT-101 | 120 | 769 |
| 17 | L iliac vein | 85.76 | tPA with MVT-101 | 110 | 500 |
| 20 | R iliac vein | -9.39 | tPA with MVT-101 | 178 | 500 |
| 21 | L iliac vein | -1.50 | tPA with MVT-101 | 179 | 500 |
| 22 | R iliac vein | 67.24 | tPA with MVT-101 | 183 | 500 |
| 15 | R iliac vein | 57.90 | MVT-101 | 99 | 0 |
| 16 | R iliac vein | 36.25 | MVT-101 | 101 | 0 |
| 21 | R iliac vein | 75.92 | MVT-101 | 98 | 0 |
| 22 | L iliac vein | 63.82 | MVT-101 | 102 | 0 |
| 23 | L iliac vein | -53.90 | MVT-101 | 191 | 0 |
| 18 | L iliac vein | 28.43 | tPA | 112 | 500 |
| 20 | L iliac vein | 63.57 | tPA | 99 | 500 |
| 24 | R iliac vein | 32.07 | tPA | 111 | 500 |
| 24 | L iliac vein | 7.99 | tPA | 111 | 500 |
| 17 | R iliac vein | -59.87 | tPA | 105 | 500 |
| 19 | R iliac vein | -149.60 | Control | 106 | 0 |
| 16 | L iliac vein | -50.49 | Control | 101 | 0 |
Parameters at baseline and post-treatment with endovascular ultrasound, MVT-101 and tPA. Data are the mean ± standard deviations, number of pigs, n =5, and * for p<0.05; ** for p<0.01 and *** for p<0.005.
| Parameters | Baseline | End of Study | p and n |
|---|---|---|---|
| Pulmonary Artery Pressure (PAP) | 21.0±2.2 | 18.7±1.3 | 0.25 |
| Pulmonary Capillary Wedge pressure (PCW) | 16.3±2.1 | 15.0±3.3 | 0.65 |
| Oxygen Saturation (sO2) | 99.7±0.5 | 99.7±0.8 | 1.00 |
| Heart Rate (Pulse Oximetry) | 95.2±9.1 | 99.2±19.4 | 0.66 |
| Mean arterial pressure (MAP) | 67.3±6.7 | 62.0±7.3 | 0.22 |
| Systolic pressure (SYS) | 90.2±7.5 | 78.5±10.3 | 0.14 |
| Diastolic pressure (DIA) | 53.2±5.3 | 49.7±5.5 | 0.33 |
Blood hematology analyses pre- and post-endovascular US treatment (n=4 pigs, * for p<0.05)
| Test | Units | Baseline | Post-treatment | p value |
|---|---|---|---|---|
| ALB | g/dL | 3.2±0.1 | 2.9±0.1 | 0.02, * |
| TP | g/dL | 5.4±0.1 | 4.8±0.6 | 0.01, * |
| ALP | U/L | 126.7±35.5 | 118.5±34.0 | 0.76 |
| ALT | U/L | 28.7±3.9 | 25.0±2.9 | 0.21 |
| AST | U/L | 18.3±1.8 | 17.0±2.2 | 0.43 |
| CK | U/L | 487.5±131.1 | 558.0±184.4 | 0.57 |
| TBIL | mg/dL | 0.15±0.08 | 0.20±0.06 | 1 |
| DBIL | mg/dL | 0.05±0.07 | 0.10±0.06 | 1 |
| BUN | mg/dL | 5.0±1.6 | 6.0±1.8 | 0.47 |
| CREAT | mg/dL | 1.3±0.2 | 1.1±0.2 | 0.42 |
| CA | mg/dL | 9.7±0.3 | 9.4±0.3 | 0.21 |
| CHOL | mg/dL | 71.5±10.4 | 67.5±6.6 | 0.58 |
| GLU | mg/dL | 125.7±21.6 | 119.5±26.5 | 0.74 |
| PHOS | mg/dL | 7.6±0.4 | 7.4±0.3 | 0.43 |
| TCO2 | mEq/L | 30.0±1.2 | 28.0±1.4 | 0.09 |
| NEUT | mEq/L | 139.3±1.9 | 138.3±2.5 | 0.57 |
| K | mEq/L | 4.8±0.3 | 4.6±0.7 | 0.59 |
| CL | mEq/L | 102.8±1.3 | 103.8±3.3 | 0.60 |
| GLOB | g/dL | 2.1±0.2 | 1.9±0.3 | 0.16 |
| A/G | Ratio | 1.5±0.2 | 1.7±0.3 | 0.52 |
| B/C | Ratio | 4.3±1.5 | 5.5±2.1 | 0.39 |
| IBIL | mg/dL | 0.10±0.07 | 0.30±0.46 | 0.37 |
| ANION | mEq/L | 11.5±1.1 | 11.5±1.3 | 1.00 |
Blood chemistry analysis pre- and post-treatment with endovascular ultrasound, with MVT-101 and tPA. Data are the mean ± standard deviations, n=4 pigs, * for p<0.05; ** for p<0.01 and *** for p<0.005.
| Test | Units | Baseline | Post-treatment | p value |
|---|---|---|---|---|
| WBC | x10³ /uL | 18.7±1.9 | 14.2±0.6 | 0.008,** |
| RBC | X106/uL | 6.2±0.5 | 5.5±0.2 | 0.09 |
| HGB | g /dL | 11.2±0.5 | 10.0±0.5 | 0.02, * |
| HCT | % | 34.4±1.5 | 30.8±1.6 | 0.02, * |
| MCV | fL | 56.1±2.7 | 55.9±3.0 | 0.91 |
| MCH | pg | 18.2±0.8 | 18.0±1.1 | 0.77 |
| MCHC | g / dL | 32.5±0.3 | 32.3±0.2 | 0.27 |
| RDW | % | 14.5±0.9 | 14.6±1.1 | 0.90 |
| PLT | x10³ /uL | 203.5±37.9 | 186.8±25.2 | 0.53 |
| MPV | fL | 8.8±0.9 | 8.1±0.6 | 0.30 |
| %NEUT | % | 26.5±2.7 | 58.5±1.3 | <0.0001,*** |
| %LYMPHS | % | 67.0±3.5 | 39.5±1.29 | <0.0001, *** |
| %MONO | % | 3.3±0.8 | 1.0±0.0 | 0.003, *** |
| %EOS | % | 3.0±0.7 | 1.3±0.5 | 0.001, *** |
| %BASO | % | 0.3±0.4 | 0.0±0.0 | 0.35 |
| %BANDS | % | 0±0 | 0±0 | NA |
| #NEUT | x10³ /uL | 4.9±0.9 | 8.3±0.3 | 0.0008, *** |
| #LYMPHS | x10³ /uL | 12.4±1.1 | 5.6±0.3 | <0.0001, *** |
| #MONO | x10³ /uL | 0.6±0.2 | 0.10±0.01 | 0.003, *** |
| #EOS | x10³ /uL | 0.6±0.2 | 0.20±0.08 | 0.001, *** |
| #BASO | x10³ /uL | 0.05±0.09 | 0.0±0.0 | 0.35 |
| #BANDS | x10³ /uL | 0±0 | 0±0 | NA |
Conclusion
The comparable efficacy seen with MVT-101 only and with tPA only in combination with the EKOS™ system may indicate that MVT-101 could provide an effective means by which to improve cavitation for the lysis of DVT in patients who would not be able to receive thrombolytic treatment. Transcutaneous ultrasound with MVT-101 and tPA was also consistently efficient at sonothrombolysis and may provide a rapid non-invasive treatment for DVT. We also note that all animals were given IV fluids throughout the procedure to form the clots and during sonothrombolysis. The fluid load and surgical stress (as encountered in the procedures in our experiments) likely causing fluid retention and causing the levels measured in the blood [43]. Finally, while preclinical safety and efficacy datasets were collected, we acknowledge that they were obtained from an animal model with acute clot. Acute clots are expected to have high porosity (~50-80%) [44, 45], a loose structure, a better drug penetration. Retracted/hard clots are on the contrary know to have low porosity (~10-30%) [46, 47], be dense and compact, and resistant to both mechanical removal and pharmacologic dissolution. We did not measure the porosity of the clots in our study. In the literature, experiments have confirmed that nanoliposomes with the size around 100 nm are small enough to diffuse freely through the fibrin mesh of thrombi as referenced by Petrokova et al [48]. PSMB that are about 1/10 as large as standard MB (average diameter 150-350 nm vs 1-3 microns), and owing to this smaller size, are able to penetrate deeper into microthrombi with nanometer scale fibrin spacing [33, 34], reach tissues beyond the vasculature and deliver medications intracellularly. It is known that the permeability of clots formed in vivo can vary by up to five orders of magnitude, with pore sizes that range from 4 to 350 nm according to Wufsus et al [34]. In reference to our nanobubbles (PSMB), it has been demonstrated that they can achieve thrombolysis of acute and hard/retracted clots [36-38]. To our knowledge, there is not an animal model that exists that has been able to reproducibly model chronic DVT. The current model for chronic DVT in pigs that has been cited has a high attrition rate [49] and would not reliably illustrate the physiology of chronic DVT.
Abbreviations
ALB: Albumin; ALP: Alkaline phosphatase; AALT: Alanine aminotransferase; AST: Aspartate aminotransferase; A/G: Albumin/Globulin ratio; ANION: Anionic; BANDS: Band Neutrophils; BASO: Basophils; B/C: Bilirubin/Creatinine ratio; BUN: Blood urea nitrogen; CA: Carbohydrate antigen; C: Creatinine; CREAT: Creatinine; CHOL: Cholesterol; CL: Total cholesterol; DIA: Diastolic Pressures; DBIL: Direct bilirubin; DVT: Deep Vein Thrombosis; EOS: Eosinophils; EVUS: Endovascular Ultrasound; GLU: glucose; GLOB: globulin; HCT: Hematocrit; HGB: Hemoglobin; IBIL: Intrahepatal bilirubin; K: Potassium; LYMPHS: Lymphocytes; MAP: Mean Arterial Pressure; MCV: Mean Corpuscular Volume; MCH: Mean Corpuscular Hemoglobin; MCHC: Mean Corpuscular Hemoglobin Concentration; MB: Microbubbles; MPV: Mean Platelet Count; MVO: Microvascular Obstruction; NEUT: Neutrophils; NLR: Neutrophil-to-Lymphocytes ratio; PA: Pulmonary Angiogram; PAP: Pulmonary Artery Pressure; PCWP: Pulmonary Capillary Wedge Pressure; PCDT: Pharmacomechanical catheter-directed thrombolysis; PE: Pulmonary Emboli; PHOS: phosphate; PLT: Platelet Count; PSMB: Phase Shift Microbubbles; PTS: Post-thrombotic Syndrome; RBC: Red Blood Cells; RDW: Red Cell Distribution Width; SYS: Systolic Pressure; sO2: Oxygen Saturation; SL: Sonothrombolysis; MONO: Monocytes; TCO2: Total carbon dioxide; TCUS: transcutaneous ultrasound; TBIL: Total bilirubin; TP: Total Protein; tPA: tissue Plasminogen Activator; US: Ultrasound; WBC: Whole Blood Cells.
Supplementary Material
Supplementary figures.
Acknowledgements
Funding was provided by the National Heart, Lung and Blood Institute (R43HL152819 and R33HL156350) to Microvascular Therapeutics, Inc. The authors thank the Boston Scientific team for providing the EKOSONIC Endovascular System, the team at Synchrony Labs and the Sonographers (Daniel Rivera, Richard Palma, and Eduardo Sandoval) for their help during animal studies.
Competing Interests
EJM, ECU, and JL acknowledge a competing interest in Microvascular Therapeutics, Inc. The other authors have declared that no competing interest exists.
References
1. https://www.cdc.gov/ncbddd/dvt/data.html
2. Goldhaber SZ, Morrison RB. Cardiology patient pages. Pulmonary embolism and deep vein thrombosis. Circulation. 2002;106:1436-8
3. Scarvelis D, Wells PS. Diagnosis and treatment of deep-vein thrombosis. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006;175:1087-92
4. Enden T, Haig Y, Kløw NE, Slagsvold CE, Sandvik L, Ghanima W. et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet (London, England). 2012;379:31-8
5. Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR. et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet (London, England). 2014;383:880-8
6. Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ. et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Annals of internal medicine. 2008;149:698-707
7. Prandoni P, Lensing AW, Prins MH, Frulla M, Marchiori A, Bernardi E. et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Annals of internal medicine. 2004;141:249-56
8. Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Annals of surgery. 2004;239:118-26
9. Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ. et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. Journal of thrombosis and haemostasis: JTH. 2008;6:1105-12
10. Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP. et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol. 2006;17:417-34
11. Cynamon J, Stein EG, Dym RJ, Jagust MB, Binkert CA, Baum RA. A new method for aggressive management of deep vein thrombosis: retrospective study of the power pulse technique. J Vasc Interv Radiol. 2006;17:1043-9
12. Hilleman DE, Razavi MK. Clinical and economic evaluation of the Trellis-8 infusion catheter for deep vein thrombosis. J Vasc Interv Radiol. 2008;19:377-83
13. Semba CP, Dake MD. Iliofemoral deep venous thrombosis: aggressive therapy with catheter-directed thrombolysis. Radiology. 1994;191:487-94
14. Vedantham S, Vesely TM, Sicard GA, Brown D, Rubin B, Sanchez LA. et al. Pharmacomechanical thrombolysis and early stent placement for iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2004;15:565-74
15. Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ. et al. Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. The New England journal of medicine. 2017;377:2240-52
16. Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419-24
17. Mangi MA, Rehman H, Bansal V, Zuberi O. Ultrasound Assisted Catheter-Directed Thrombolysis of Acute Pulmonary Embolism: A Review of Current Literature. Cureus. 2017;9:e1492
18. Khan K, Yamamura D, Vargas C, Alexander T, Surani S. The Role of EkoSonic Endovascular System or EKOS® in Pulmonary Embolism. Cureus. 2019;11:e6380
19. Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. The New England journal of medicine. 2002;347:1143-50
20. Tapson VF, Sterling K, Jones N, Elder M, Tripathy U, Brower J. et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovascular interventions. 2018;11:1401-10
21. Bader KB, Gruber MJ, Holland CK. Shaken and stirred: mechanisms of ultrasound-enhanced thrombolysis. Ultrasound Med Biol. 2015;41:187-96
22. Cui H, Zhu Q, Gao Y, Xia H, Tan K, He Y. et al. Ultrasound Mediated Microbubbles Destruction Augmented Sonolysis: An In Vitro and In Vivo Study. BioMed research international. 2017;2017:7021929
23. Kim J, DeRuiter RM, Goel L, Xu Z, Jiang X, Dayton PA. A Comparison of Sonothrombolysis in Aged Clots between Low-Boiling-Point Phase-Change Nanodroplets and Microbubbles of the Same Composition. Ultrasound in medicine & biology. 2020;46:3059-68
24. Slikkerveer J, Dijkmans PA, Sieswerda GT, Doevendans PA, van Dijk AP, Verheugt FW. et al. Ultrasound enhanced prehospital thrombolysis using microbubbles infusion in patients with acute ST elevation myocardial infarction: rationale and design of the Sonolysis study. Trials. 2008;9:72
25. Porter T, Kricsfeld D, Li S, Bonich T. Comparison of D.dimer activity and residual particle size produced floowing thrombus disruption by perfluorocarbon containing microbubbles versus urokinase in the presence of low frequency ultrasound.. JAmColl Cardiol. 31: 21-219
26. Kim J, Lindsey BD, Chang WY, Dai X, Stavas JM, Dayton PA. et al. Intravascular forward-looking ultrasound transducers for microbubble-mediated sonothrombolysis. Scientific reports. 2017;7:3454
27. Kutty S, Wu J, Hammel JM, Xie F, Gao S, Drvol LK. et al. Microbubble mediated thrombus dissolution with diagnostic ultrasound for the treatment of chronic venous thrombi. PloS one. 2012;7:e51453
28. Ebben HP, Nederhoed JH, Lely RJ, Wisselink W, Yeung K. Microbubbles and UltraSound-accelerated Thrombolysis (MUST) for peripheral arterial occlusions: protocol for a phase II single-arm trial. BMJ open. 2017;7:e014365
29. Doelare SAN, Jean Pierre DM, Nederhoed JH, Smorenburg SPM, Lely RJ, Jongkind V. et al. Microbubbles and Ultrasound Accelerated Thrombolysis for Peripheral Arterial Occlusions: The Outcomes of a Single Arm Phase II Trial. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2021;62:463-8
30. Alkarithi G, Duval C, Shi Y, Macrae FL, Ariëns RAS. Thrombus Structural Composition in Cardiovascular Disease. Arteriosclerosis, thrombosis, and vascular biology. 2021;41:2370-83
31. Mohammed SA, Amjad MW, Acosta MF, Chen X, Lavery L, Hanrahan D. et al. Fibrin-targeted phase shift microbubbles for the treatment of microvascular obstruction. Nanotheranostics. 2024;8:33-47
32. Ząbczyk M, Ariëns RAS, Undas A. Fibrin clot properties in cardiovascular disease: from basic mechanisms to clinical practice. Cardiovascular research. 2023;119:94-111
33. Voter WA, Lucaveche C, Blaurock AE, Erickson HP. Lateral packing of protofibrils in fibrin fibers and fibrinogen polymers. Biopolymers. 1986;25:2359-73
34. Wufsus AR, Macera NE, Neeves KB. The hydraulic permeability of blood clots as a function of fibrin and platelet density. Biophysical journal. 2013;104:1812-23
35. Kim J, Bautista KJB, Deruiter RM, Goel L, Jiang X, Xu Z. et al. An Analysis of Sonothrombolysis and Cavitation for Retracted and Unretracted Clots Using Microbubbles Versus Low-Boiling-Point Nanodroplets. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2022;69:711-9
36. El Kadi S, Qian L, Zeng P, Lof J, Stolze E, Xie F. et al. Efficacy of Sonothrombolysis Using Acoustically Activated Perflutren Nanodroplets versus Perflutren Microbubbles. Ultrasound in medicine & biology. 2021;47:1814-25
37. Goel L, Wu H, Zhang B, Kim J, Dayton PA, Xu Z. et al. Nanodroplet-mediated catheter-directed sonothrombolysis of retracted blood clots. Microsystems & nanoengineering. 2021;7:3
38. Goel L, Wu H, Zhang B, Kim J, Dayton PA, Xu Z. et al. Safety Evaluation of a Forward-Viewing Intravascular Transducer for Sonothrombolysis: An in Vitro and ex Vivo Study. Ultrasound in medicine & biology. 2021
39. Schwein A, Magnus L, Markovits J, Chinnadurai P, Autry K, Jenkins L. et al. Endovascular Porcine Model of Iliocaval Venous Thrombosis. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2022;63:623-30
40. Hu C, Zhao B, Ye Q, Zou J, Li X, Wu H. The Diagnostic Value of the Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio for Deep Venous Thrombosis: A Systematic Review and Meta-Analysis. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2023;29:10760296231187392
41. Yu FTH, Chen X, Straub AC, Pacella JJ. The Role of Nitric Oxide during Sonoreperfusion of Microvascular Obstruction. Theranostics. 2017;7:3527-38
42. Sheeran PS, Rojas JD, Puett C, Hjelmquist J, Arena CB, Dayton PA. Contrast-enhanced ultrasound imaging and in vivo circulatory kinetics with low-boiling-point nanoscale phase-change perfluorocarbon agents. Ultrasound Med Biol. 2015;41:814-31
43. Desborough JP. The stress response to trauma and surgery. British journal of anaesthesia. 2000;85:109-17
44. Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI. et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123:1596-603
45. Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thrombosis and haemostasis. 2009;102:1169-75
46. Duffy S, Farrell M, McArdle K, Thornton J, Vale D, Rainsford E. et al. Novel methodology to replicate clot analogs with diverse composition in acute ischemic stroke. Journal of neurointerventional surgery. 2017;9:486-91
47. Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie C. et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. Journal of neurointerventional surgery. 2017;9:529-34
48. Petroková H, Mašek J, Kuchař M, Vítečková Wünschová A, Štikarová J, Bartheldyová E. et al. Targeting Human Thrombus by Liposomes Modified with Anti-Fibrin Protein Binders. Pharmaceutics. 2019 11
49. Wang C, Tang T, Ye SL, Hu N, Du XL, Li XQ. Comparison between canine and porcine models of chronic deep venous thrombosis. Thrombosis journal. 2023;21:121
Author contact
![]() Corresponding author: Emmanuelle J Meuillet, PhD, Microvascular Therapeutics Inc, 1635 East 18th street, Tucson AZ 85719; Email: e.meuilletcom; Telephone: 520-730-3264; Website: http://mvtpharma.com
Corresponding author: Emmanuelle J Meuillet, PhD, Microvascular Therapeutics Inc, 1635 East 18th street, Tucson AZ 85719; Email: e.meuilletcom; Telephone: 520-730-3264; Website: http://mvtpharma.com

 Global reach, higher impact
Global reach, higher impact