ISSN: 2206-7418
Nanotheranostics 2021; 5(4):378-390. doi:10.7150/ntno.54879 This issue Cite
Research Paper
Engineering Extracellular Vesicles to Target Pancreatic Tissue In Vivo
1. Department of Cardiovascular Medicine, Tokyo Medical and Dental University, Tokyo, Japan.
2. Institute for Quantitative Health Science and Engineering (IQ), Michigan State University, Michigan, USA.
3. Lyman Briggs College, Michigan State University, Michigan, USA.
4. Department of Chemical Engineering and Material, Michigan State University, Michigan, USA.
5. Department of Microbiology and Molecular Genetics, Michigan State University, Michigan, USA.
6. Department of Biomedical Engineering, Michigan State University, Michigan, USA.
* These authors contributed equally to this work.
Abstract
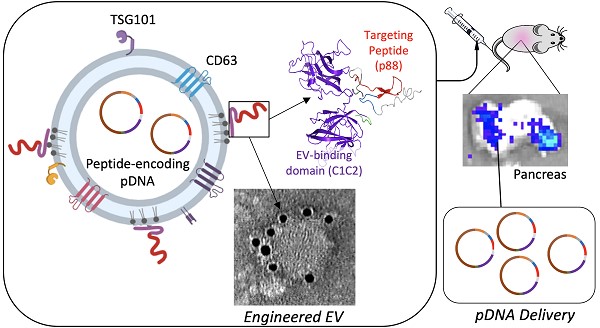
Extracellular vesicles (EVs) are naturally released, cell-derived vesicles that mediate intracellular communication, in part, by transferring genetic information and, thus, have the potential to be modified for use as a therapeutic gene or drug delivery vehicle. Advances in EV engineering suggest that directed delivery can be accomplished via surface alterations. Here we assess enriched delivery of engineered EVs displaying an organ targeting peptide specific to the pancreas. We first characterized the size, morphology, and surface markers of engineered EVs that were decorated with a recombinant protein specific to pancreatic β-cells. This β-cell-specific recombinant protein consists of the peptide p88 fused to the EV-binding domain of lactadherin (C1C2). These engineered EVs, p88-EVs, specifically bound to pancreatic β-cells in culture and transferred encapsulated plasmid DNA (pDNA) as early as in 10 min suggesting that the internalization of peptide-bearing EVs is a rapid process. Biodistribution of p88-EVs administrated intravenously into mice showed an altered pattern of EV localization and improved DNA delivery to the pancreas relative to control EVs, as well as an accumulation of targeting EVs to the pancreas using luciferase activity as a readout. These findings demonstrate that systemic administration of engineered EVs can efficiently deliver their cargo as gene carriers to targeted organs in live animals.
Keywords: Extracellular Vesicles, Pancreatic β-cells, EVs engineering, Imaging, Targeted delivery.

 Global reach, higher impact
Global reach, higher impact