ISSN: 2206-7418
Nanotheranostics 2025; 9(3):262-279. doi:10.7150/ntno.113606 This issue Cite
Review
Plant-Derived Natural Product-Based Nanoformulations for Healthcare Application
1. CSIR-Centre for Cellular and Molecular Biology (CCMB), Uppal Road, Hyderabad, Telangana, India.
2. Department of Physiology, College of Medicine, University of Tennessee Health Science Center, Memphis, TN, USA.
3. Manipal Center for Biotherapeutics Research, Manipal Academy of Higher Education, Karnataka, Manipal, 576 104, India.
4. Department of Biotechnology, All India Institute of Medical Sciences (AIIMS), New Delhi, India.
* These authors have made equal contribution.
Received 2025-3-11; Accepted 2025-6-15; Published 2025-8-16
Abstract
Plants produce numerous natural compounds developed into commercial products. These compounds offer medicinal benefits for treating diseases like diabetes, neurological disorders, malaria, and cancer. They also serve as hepatoprotective agents and immunomodulators. These natural products are secondary metabolites that plants produce for their defense and adaptation. Recently, numerous reports have highlighted the effectiveness of natural products in different diseases. However, comprehensive analysis of plant-based products currently used in the clinical setting for various human diseases is insufficient. This review provides extensive information about the application of natural plant products in both clinical and preclinical settings. It highlights their role in developing drugs for human diseases. Additionally, these plant products could serve as diagnostic tools for various diseases. Plant-derived natural products, integrated with advanced nanotechnology-based approaches, could enhance healthcare monitoring without compromising treatment efficacy. Nanotechnology techniques employing both diagnostics and therapeutics, known as nanotheranostics, utilize engineered biocompatible nanomaterials with potential prospects for healthcare management. Nanomaterials like polymeric nanoparticles and liposomes offer diagnostic value by enabling real-time imaging of disease progression and treatment response. Functionalized with contrast agents or dyes, they enhance MRI, CT, PET, and fluorescence imaging, improving diagnosis, patient stratification, and monitoring of drug delivery and efficacy. With the increasing demand for natural dietary supplements, this issue encompasses the identification of various plant-based natural products as potential nanotheranostics with promising potential for chronic disorders such as cancer, neurological pathologies, diabetes, and immunological issues. This review focuses on applications of nanotheranostics utilizing natural products in biomedical applications, outlining the current breakthroughs, supplemented with future potentialities.
Keywords: Natural products, Nanotheranostics, Healthcare, Nanoparticles, Diabetes, Cancer, Immunomodulation.
Introduction
India is home to over 45,000 plant species, with around 20,000 recognized for their medicinal potential. Of these, approximately 7,500 are used by traditional communities in healthcare practices, representing the highest percentage of medicinal plant use globally [1, 2]. Ayurveda, India's ancient medical system, identifies around 2,000 such plants, while Siddha and Unani recognize 1,121 and 751, respectively. Roughly 25% of all drugs are plant-derived [3]. In contrast, modern western medicine remains inaccessible and unaffordable for many worldwide. Despite over a century of western medical influence in India, traditional medicine continues to serve as the primary healthcare resource for a large population. The World Health Organization (WHO) supports the use of indigenous systems based on locally available medicinal plants. Notably, about 50% of drugs in the United States contain one or more natural products [4]. Natural compounds are generally classified into primary and secondary metabolites. Primary metabolites—such as nucleic acids, amino acids, sugars, and fatty acids—are essential for basic life processes. Secondary metabolites, on the other hand, are bioactive compounds that support ecological functions and often provide therapeutic benefits. These are extracted from diverse biological sources including plants, marine organisms, and microorganisms. Within raw extracts, novel bioactive molecules—referred to as active ingredients—can be identified. Many plant-derived secondary metabolites demonstrate therapeutic benefits for a range of diseases. To enhance their effectiveness and overcome delivery limitations, researchers are increasingly applying nanotechnology-based solutions. Nanotheranostics refers as integrated nanotechnology platform that combines diagnostic and therapeutic functionalities within a single system, enabling simultaneous imaging, targeted drug delivery, and real-time monitoring of treatment efficacy [5]. These multifunctional systems allow for the targeted delivery of both diagnostic agents and therapeutics, offering a significant advantage over traditional methods. They have shown promise in managing various acute and chronic conditions, including cancer, diabetes, neurological disorders, and immune-related diseases [6]. To address these challenges, plant-derived nanomaterials—such as nanoparticles, lipid-based carriers, magnetic conjugates, silica encapsulates, and carbon-based structures—act as effective nanotheranostic agents. These nanotechnology-fused plant-based nanomedicines, nanodrugs, nanophenols, the green synthesis of nanoparticles, etc., display improved potential for detecting and targeting specific body tissues/cells with promising therapeutic potential [7]. Nanotechnology plays a crucial role in boosting the performance of herbal medicines, especially those with poor solubility, low intestinal permeability, rapid metabolic breakdown, or limited systemic absorption. Nanoformulations help address these issues, ensuring more effective delivery to target sites [8]. These nanoforms can also be utilized for diagnosis along with treating disease. This involves delivering diagnostic agents to specific targets and subsequently visualizing their distribution by imaging techniques [9-11]. Nanoparticles' large surface area enables them to carry multiple targeting ligands, imaging probes, and therapeutic agents, providing a flexible platform for noninvasive diagnostics and enhancing diagnostic accuracy through various imaging modalities [12]. Chemical and biological markers, when linked to nanoparticles, offer a flexible platform for noninvasive diagnostics. These markers provide both qualitative and quantitative data through imaging modalities such as Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Positron Emission Tomography (PET), and Near-infrared (NIR). These imaging techniques can respond to variety of stimulus, such as magnetic fields, temperature, ultrasound, electric pulses, and internal factors such as glucose levels, pH, and redox conditions, increasing diagnostic accuracy and patient comfort [13]. The draft uniquely consolidates recent advancements in the design and application of plant-derived natural product-based nanoformulations across diverse healthcare domains, as represented in Fig. 1. A diverse range of nanotheranostic agents, have been applied to develop natural product-based nanotheranostics for healthcare [14]. In addition, reports and studies related to the use of plant-based exosomes, micelles, nanovesicles, nanoscaffolds, etc., as nanotheranostic agents for biomedical disorders are elaborately discussed and illustrated in Fig. 2. This review also discusses the different nanotheranostic variants of plants and their secondary metabolites, which offer a wide range of advantages over conventional and synthetic nanotechnology for biomedical management, as graphically compiled in Fig. 3. Unlike existing literature that often focuses on individual classes of phytoconstituents or specific diseases, our review offers a comprehensive perspective spanning phytochemical diversity, nanocarrier systems, and translational relevance. By integrating insights into formulation strategies, therapeutic mechanisms, and clinical promise, this review aims to bridge the gap between phytomedicine and precision nanotheranostics.
Natural product-based nanotheranostics for treating diabetes
Diabetes is a significant health problem that is associated with a high risk of various complications. Hyperglycemia is characterized by elevated blood glucose levels. Persistent hyperglycemic illness leads to neuropathy, stroke, nephropathy, and cardiovascular disease (CVD) in the context of Type 2 Diabetes Mellitus (T2DM). As a result, controlling blood glucose levels is a crucial approach for the prevention of T2DM. Herbs, beans, and onions contain polyphenols, which improve digestive issues, neurodegenerative disease, cardiovascular complications, and diabetes [15]. One report suggested that onion has anti-inflammatory, antioxidant, and antidiabetic effects because of the presence of flavonoid like quercetin and organosulfur compounds [16-18]. The Amadori rearrangement compound obtained from heat-processed onion extract suppressed the absorption of carbohydrates by blocking intestinal sucrose, thereby reducing blood glucose levels [19]. Cannabinoid receptor 1 (CB1R) activation exacerbates insulin resistance and hyperglycemia by inducing endoplasmic reticulum stress and gluconeogenesis. Gomisin, a compound from Schisandra chinensis, ameliorates CB1R-mediated insulin dysfunction and glucose metabolism abnormalities in vitro. In vivo studies demonstrated that gomisin attenuates hyperglycemia induced by a high-fat diet, insulin resistance, and endoplasmic reticulum (ER) stress by inhibiting CB1R signaling and ceramide biosynthesis [20]. A few examples of plant products that exhibit antidiabetic properties are discussed in Table 1 below. The field of nanotheranostic strategies for diabetes management is rapidly advancing and holds the potential to transform diabetes management. By combining diagnostics and therapeutics with a single nanoparticle, this approach enables targeted drug delivery, improved glucose monitoring, and enhanced wound healing. Nanotheranostics offer promising prospects for improving the lives of people with diabetes [21]. Various studies have reported polymeric nanoparticle-based delivery of insulin via oral and nasal routes. This affects treatment physiology via age-related metabolic variations that in turn influence the bioavailability and release of insulin [22]. One dietary bioflavonoid, quercetin, has been studied as a superparamagnetic iron oxide nanoparticle conjugated with quercetin (QCSPIONs) for reducing blood glucose levels in diabetic rat models. The authors demonstrated that the nanoconjugate-driven regulation of microRNA-29, which elevates the expression of glucose transporters and insulin-like growth factor-1, plays a crucial role in preventing diabetic complications [23, 24]. Cheng et al. (2020) reported a green synthesis method for producing gold nanoparticles using an extract from Ramulus mori plant leaves and methanol. The plant extract was conjugated onto polyacrylic gold nanoparticles, which were developed as a novel drug delivery system for gestational diabetes. A large variety of nanoformulations have been fabricated for diabetes, ensuring efficient targeted drug delivery with the required release system. Additionally, targeted ligand-based delivery via nanocarriers increases drug availability and stability, thus reducing the dosage and administration frequency [25].
Natural product-based nanotheranostics for treating neurodegenerative diseases
Neurodegenerative diseases are significant issues impacting millions of people globally [26]. These diseases are marked by the degeneration of neurons in the central nervous system, affecting particular brain functions. The most notable neurodegenerative disorders include Huntington's disease (HD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), Alzheimer's disease (AD), multiple sclerosis (MS). The origin and pathophysiology of these neurodegenerative diseases are unspecified; however, it is widely believed that these disorders have common underlying causes at the cellular and molecular level, including: oxidative stress, chronic inflammation, mitochondrial dysfunction, disrupted calcium balance, and protein misfolding [27-29].
Schematic illustration displaying various diseases for which plant-based natural products and their nanotheranostics are discussed in the current report.
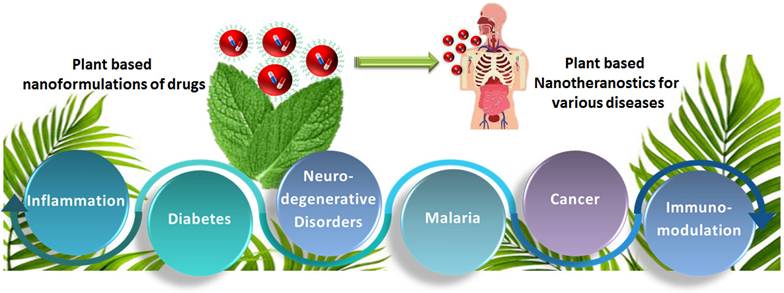
Various nanotheranostic agents, such as nanomaterials as metallic and polymeric nanoparticles and other biological nanosystems, that are utilized to design plant-based nanotheranostics for healthcare applications.
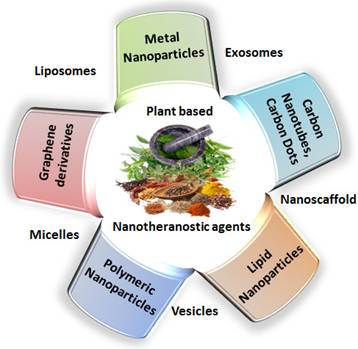
A diagrammatic compilation of different advantages offered by plant product-based nanotheranostics (left) along with various nanotheranostic variants that are designed from plants and their natural products.
Plant products that regulate blood sugar levels and control diabetes
| Plant Product | Importance in Health | Mechanism of Action |
|---|---|---|
| Cannabinoid type 1 receptor (CB1R) | Stimulates endoplasmic stress, contributing to insulin resistance and increased gluconeogenesis. | High fat diet (HFD)-induced increased blood glucose and insulin level and downregulation of HFD-induced ER stress, gluconeogenic gene, expression of CB1R, and ceramide synthesis [20] |
| Onion | Exhibits anti-inflammatory, antioxidant, and antidiabetic values due to flavonoids compounds such as quercetin and organosulfur compounds | The Amador rearrangement compound suppressed the absorption of carbohydrates by blocking the intestinal sucrose [141] |
| Myricitrin | Antioxidant, anti-diabetic, and antiapoptotic effects in mice and myotube cells | Myricitrin-loaded SLNs enhanced β-cell function, improving hyperglycemia through increased insulin levels [142] |
| Aqueous bark extract of Saraca asoca | Testing wound healing efficacy by studying the expression of inflammatory factors like cytokines and pro-inflammatory markers in diabetic mice | Green-synthesized silver nanoparticles effectively killed multidrug-resistant bacteria, showing promise as a safe topical treatment for diabetic wounds [143] |
| Oleanolic acid and Polygalacturonic acid | Anti-diabetic properties | The nanocomplex micelles promoted IRS-1/PI3K/AKT signaling pathway via inhibition of PTP1B enzyme [144] |
| Stevia Glycosides | Anti-diabetic nanoformulation | Oral bioavailability and target specificity; Nanotheranostic agent as Stevioside-assembled Polyethylene glycol nanoparticles [145] |
| Mulberry leaf and Pueraria Lobata extracts | Hypoglycemic effects | Slow drug release and Intestinal permeability; Nanotheranostic agent as Mulberry leaf and Pueraria Lobata extracts loaded selenium-layered nanoparticles [146] |
| Emodin | Diabetic management | Attenuate diabetes-provoked neuropathic pain via suppressing purin 2X3 (P2X3) receptor expression; Nanotheranostic agent as Emodin-loaded poly-PEGMA-DMAEMA-MAM nanoparticles [147] |
Important role of plants produced in neurodegenerative disorders
| Plant Product | Neurodegenerative Disease | Mechanism of Action |
|---|---|---|
| Lippia citriodora extract and verbascoside | PD, HD and AD | Regulating genes responsible for maintaining calcium homeostasis and energy production, enhancing the expression of serotonin, brain-derived neurotrophic factor (BDNF), and dopamine [30] |
| Auraptene (found in citrus fruit) | PD | Inhibiting mitochondrial respiration and reducing ROS generation [32] |
| Chionanthus retusus extract | PD, HD, AD, MS and ALS | Blocking LPS-induced NO production and neuroprotection against cellular toxicity induced by glutamate [148] |
| Centella asiatica extract | AD | Attenuating cognitive decline, inhibiting morphological aberration in the hippocampus, decreasing the level of glycogen synthase kinase-3 beta, and increasing protein phosphatase 2 level [149] |
| Thymol (found in various plants) | PD | Attenuating oxidative stress, neuronal loss, and inflammation in a rat model of Parkinson's disease induced by rotenone [150] |
Plant product-derived nanotheranostics for the control of neurodegenerative disorders
| Plant Product | Importance in Health | Mechanism of Action | Nanotheranostic agent |
|---|---|---|---|
| Chitosan-gelatin-green tea extract | PD | Generation of reactive oxygen species, increased expression of tyrosine hydroxylase (TH) enzyme, and decreased expression of α-syn protein. | Chitosan-gelatin-green tea extract nanoparticles [151, 152] |
| Ephedra sinica Stapf extract | Neuroinflammation | Anti-neuroinflammatory properties | Gold nanoparticle [153] |
| Ferulic acid (FA) | AD | Exhibits antioxidant, anticancer, cardioprotective, and neuroprotective properties. | Chitosan-coated solid lipid nanoparticles [154] |
| Berberine | AD | Suppressed AChEI activity and mitigated memory loss. | Multi walled Carbon Nanotubes coated with phospholipid and polysorbate [155] |
| Retinoic acid | PD | Inhibit α-syn aggregation in vitro | Chitosan nanoparticles and nanomicelle [154] |
Unfortunately, in this context, there are no therapeutic interventions available to halt the progression of these diseases. However, various studies have shown that natural products can be beneficial in treating neurodegeneration, which may be promising candidates for potential therapies. For example, extracts isolated from Lippia citriodora (VEE) and verbascoside (Vs), which are phenylpropanoid glycosides, exert relaxation effects by regulating genes responsible for maintaining calcium homeostasis and energy production [30]. An extract isolated from Chionanthus retusus blocked nitric oxide (NO) production induced by lipopolysaccharide (LPS) and showed neuroprotective effects against glutamate-induced cellular toxicity [31]. Moreover, auraptene (AUR), a 7-geranyloxylated coumarin isolated from citrus fruit, mitigated PD symptoms by inhibiting mitochondrial respiration and reducing reactive oxygen species (ROS) generation [32]. Table 2 comprises studies describing various plants that are effective for treating neurodegenerative disorders. Nanotechnology solutions for brain-related disorders employ specially designed materials and devices that operate within biological systems at the molecular level. Nanoconjugates derived from medicinal plants and their extracts have played a crucial role in the advancement of innovative neurodiagnostic and therapeutic approaches, offering potential alternatives to plant-based nanotheranostics. Their advantages include their natural abundance, targeted delivery of specific molecules to the brain, and enhanced efficacy with a decreased likelihood of side effects [33]. The efficacy and pharmacokinetic properties of plant products combined with nanoparticle-based delivery systems are enhanced. These systems can traverse the blood-brain barrier (BBB) as nanotheranostic agents for PD have been shown to be enhanced [34]. Marine algae have been extensively investigated for their wealth of bioactive nutraceuticals. Various biological and environmental factors influence the production of primary and secondary metabolites. It serves as an efficient biofactory for various theranostic compounds, and its alignment with nanomaterials is easy to handle, has the capacity to conjugate inorganic metallic ions, has a lower cost and, most importantly, is an eco-friendly, healthier synthesis of NPs. Marine algae-based nanotheranostics are excellent stabilizing agents for the green synthesis of thermodynamically stable nanoparticles [35]. Another study related to nanoparticles containing curcumin, piperine along with glyceryl monooleate (GMO) demonstrated effective penetration and sustained release into brain tissue across the BBB, indicating an anti-Parkinsonism effect. A previous study in PD model mice revealed that rotenone impaired coordination of motor neurons and controlled dopaminergic neuronal degeneration [36]. They also prevent the aggregation of α-synuclein, death of dopaminergic neurons and mitochondrial dysfunction [34]. Additionally, it has been found that lipid nanoparticles can improve the absorption and utilization of plant-based nutraceuticals, owing to their ease of administration via oral, nasal, parenteral and topical routes [37]. Many such reports are described in Table 3 below, employing nanotheranostic agents for neurodegenerative disorders.
Natural product-based nanotheranostics for Malaria
Malaria is a life-threatening and endemic disease transmitted by the bite of the Anopheles mosquito [38]. It is more prevalent in tropical countries such as Asia, Africa, and Latin America. Malaria remains a global health challenge, affecting nearly half the world's population. The World Health Organization estimated that 229 million people contracted malaria in 2019, resulting in 409,000 deaths. Plasmodium parasites cause this disease, while Plasmodium falciparum being the deadliest strain [39]. Malaria disproportionately affects young African children and hinders economic growth. Drug resistance complicates treatment and control efforts [40]. Hence, there is an urgent need for novel and herbal antimalarial products obtained from medicinal plants. The first compound in this category is quinine, which was isolated from Cinchona spp. and is still used as an effective antimalarial drug. Studies suggest that quinine, interfere with hemoglobin digestion during the blood stage of malaria infection. Despite the resistance of P. falciparum to quinine derivatives, quinine is routinely used to manage Plasmodium infection [41]. The second herbal antimalarial drug, artemisinin (ARS), was first derived from the traditional Chinese medicine Artemisia annua [42]. The structural backbone comprises a sesquiterpene lactone having a peroxide bridge that possesses excellent antimalarial activity and is also active against quinine-resistant P. falciparum [43, 44]. Furthermore, Artemisinin-based Combination Therapies (ACTs) are reported to provide better efficacy in the case of multidrug-resistant malaria [44, 45]. Subsequently, several alkaloid compounds isolated from various medicinal plants were reported to have antimalarial activity. These alkaloids possess profound structural heterogeneity and include indoles, aporphines, steroids, terpenoids, phenanthroindolizidines, hasubanane, isoquinoline, benzylisoquinoline, naphthoisoquinoline, morphinandienone, protoberberine, amaryllidaceae, cyclopeptides, quinolines, pyridocoumarins, acridones, and macrocyclic alkaloids [40]. An Indian study described sixty-eight plant species belonging to 33 families used to eradicate malarial infection in northeast India [46]. These plants either kill the parasite directly or act as a hepatic shield when combined with other plant species. Some commonly used antimalarial plant species in northeast India include Coptis teeta, Ocimum sanctum, Vitex peduncularis, Alstonia scholaris, Crotalaria occulta, and Polygala persicariaefolia. In addition, few plant species, such as Eucalyptus globule, Ocimum gratissimum, Homalomena aromatica, and Elsholtzia blanda, are described as mosquito repellents. However, whether these plant species act as repellents, insecticides or both has yet to be determined. Furthermore, several studies highlighted the effectiveness of plant-based repellents in preventing malaria [47, 48]. Plant-based medicines have exhibited promising effectiveness when combined with nanotechnology-based delivery systems, leading to better management of malarial infections [49]. Various plant parts, like leaves, roots, latex, seeds, and secondary metabolites, have been used as eco-friendly candidates for the green synthesis of nanoparticles. Plants and their natural products act as sources of reducing molecules for bio-nanosynthesis, contributing to cost-effectiveness and reduction of any harmful chemical wastes. The green-synthesis of Ag-nanoparticles using Bruguiera cylindrical, Centroceras clavulatum, and Moringa oleifera has been reported to have antimalarial and antidengue effects [50]. Ramanibai et. al., conducted a study on the larvicidal efficacy of silver nanoparticles synthesized from 2,7-bis[2-[diethylamino]-ethoxy] fluorene, isolated from Melia azedarach leaves, against Culex quinquefasciatus and Aedes aegypti. This method was adopted as a novel alternative that can be employed for mosquito control [51]. Suman et. al., explored how well titanium dioxide nanoparticles, derived from Morinda citrifolia root extract, could eliminate mosquito larvae. They tested these nanoparticles on three mosquito species—Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus—while also evaluating their safety for non-target fish [52]. Table 4 lists various herbal plants and their components employed to design nanotheranostic platforms for controlling malaria and parasitic infections.
Natural product-based nanotheranostics for cancer
Cancer is a complicated disease marked by the abnormal and uncontrolled growth and spread of cells. As a major global health issue, it is the 2nd most common cause of death worldwide, surpassed only by CVD. With over 100 identified types, cancers are classified on the basis of their origin. Lung, prostrate and colorectal cancers are the most frequently occurring cancers among men, whereas breast, lung, and colorectal cancers are the leading types in women [53]. In 2020, the total number of cancer cases was 19.3 million, accounting for 10.0 million deaths globally (GLOBOCAN 2020 database). Furthermore, the burden of cancer is increasing worldwide because of poor diagnosis and management. This global surge in cancer cases has substantially affected the country's economic status and individual physical and emotional well-being. Hence, the cancer burden can be reduced by early diagnosis and appropriate management of the patient. One approach for cancer management is chemoprevention, which involves the use of synthetic, natural molecules (such as plant extracts) and biological agents to prevent carcinogenesis at one of the steps of tumor progression: initiation, promotion, and progression [54, 55]. Medicinal plants harbor a mixture of bioactive compounds with potent anticancer activity and have been used in cancer treatment for centuries [56, 57]. The daily consumption of coffee is very high worldwide. The chemoprotective role of coffee has been established for a variety of cancers. This role can be imparted by various compounds, such as caffeine, diterpenes, and chlorogenic acid, present in coffee [54]. Since coffee is a mixture of several compounds, the exact molecular mechanism of individual compounds and other confounding factors is yet to be determined. High intake of dietary carotenoids, including β-carotene, curcumin, lutein, phytoene, crocin, crocetin, lycopene, β-cryptoxanthin, and astaxanthin, has been reported to reduce the risk of breast, ovarian, cervical, and colorectal cancers [58, 59]. Different carotenoids tend to target different pathways to regulate cancer progression. The molecular mechanisms underlying the chemoprotective function of carotenoids include apoptotic induction, targeting of gap junction intercellular crosstalk, regulating cell cycle progression, exerting antiproliferative effects, reducing the number of Bcl-2 and Bcl-xl positive cells, modulating growth factor signaling, antioxidant response elements and regulating the expression of differentiation-related proteins [58]. Resveratrol is a natural nonflavonoid polyphenol present in grape skin, peanuts, Polygonum cuspidatum, and other plants and fruits. Resveratrol possesses robust antitumor activity and has been implicated in managing various cancers, including skin, liver, breast, colon cancer [60, 61]. In a recent study, resveratrol, when used as a combination therapy with 5-FU (fluorouracil) against colon cancer cell lines (SW480 and LoVo), was shown to reduce drug resistance by modulating apoptosis through the BAX gene [61]. Resveratrol was found to suppress the metastasis of human gastric cancer cells by inhibiting the metastasis-associated lung adenocarcinoma transcript 1 (MALAT1)-mediated epithelial-mesenchymal transition (EMT) in the BGC823 cell line [62]. Green tea is a rich source of polyphenols, particularly epigallocatechin-3-gallate (EGCG). The active biomolecule EGCG has been implicated in various cancers, including prostate, brain, bladder, and cervical cancers [63, 64]. Experimental evidence from human hepatoma cell lines (HepG2 and Huh7) and hepatocellular carcinoma (HCC) rat models treated with EGCG suggested that EGCG inhibited cell growth and prolonged the lifespan of rats. by increasing the levels of p21waf1/Cip1 and downregulating CDC25A levels [65] Furthermore, several case‒control, epidemiological, and meta-analyses have been shown to reduce the risk of prostate cancer in a cohort with high tea intake [66]. Accumulating evidence from in vitro and in vivo experiments also suggests that EGCG can induce cancer cell apoptosis via epigenetic regulation of apoptosis-associated genes such as human telomerase reverse transcriptase (hTERT) or by increasing the levels of ROS [67]. A wide range of natural products with anticancer properties are listed in Table 5. The field of cancer nanotheranostics has utilized nanoparticles because of their capacity to accumulate at tumor locations as substitutes for conventional chemotherapy approaches and medical treatments. Compared with chemotherapy, natural compounds seem to offer a choice with fewer adverse effects; however, concerns about their bioavailability persist. NPs combined with medicinal and herbal plants are used as drug and gene transporters to tumor sites, as imaging agents offering reduced toxicity and effective biocompatibility. The small size of plant nanoconjugates can easily negate side effects after their administration into tumor tissues, prolonging their sustained release and enhancing their effectiveness. Moreover, coupling natural product nanoconjugates with specific receptor moieties can result in active targeting of tumor tissues [68]. Chelora et al. studied the chemotherapeutic effect of piperine extract from pepper (Pip) via self-assembly and the formation of nanoparticles with PEG (Pip NPs). Pip NPs exhibit excellent cancer-killing properties and have emerged as cost-effective anticancer herbal nanotheranostics. Carbon dots (CDs), a major group of nanotheranostic agents, have been extensively reported for their green synthesis via various plant-based materials, such as garlic, fruit extract, mulberry, oils, and Buchanania lanzan, as substrate precursors [69]. The medicinal plant-based synthesis of CDs is a one-pot, cost-effective synthesis with highly tunable properties, and its biocompatibility has attracted the attention of many researchers [70]. Fresh ginger juice has also been reported to suppress the growth of human hepatocellular carcinoma cells (HepG2) via the production of reactive oxygen [71, 72] Carbon nanotubes (CNTs), another class of nanotheranostic agent, can be produced using diverse natural hydrocarbon precursors, such as plant extracts (including tea-tree extracts), essential oils (including sunflower oils, eucalyptus oil), milk, honey, biodiesel, eggs, and other materials [73]. Other examples of such plant-based anticancer nanotheranostics are listed in Table 6.
Natural product-based nanotheranostics for immunomodulation
The immune system plays a vital role in protecting individuals from diseases and toxins. An altered or compromised immune function is associated with several conditions. Immunomodulators possess the ability to modulate immune function. Immunomodulators can be synthetic (such as chemical compounds) or natural (such as plant products) [3]. Plant-based natural immunomodulators have been explored extensively in the past few decades. Interestingly, their use is rooted in traditional medical systems and is now implicated in various pathological conditions. Herbal immunomodulators can exert their effects by directly acting on the pathogen or indirectly by modulating the immune system, i.e., both native and acquired immunity, of the host. Broadly, immunomodulators can be categorized into three major classes: a) immunostimulatory agents, b) immunosuppressive agents, and c) tolerogens [3]. Immunostimulatory molecules are used in cases of immunodeficiency, infections, and cancers and act by stimulating or augmenting the molecules of the immune system. In contrast, immunosuppressive agents are used in autoimmune diseases or transplantation and work by weakening the immune system. Tolerogens tend to confer immunological tolerance and make the immune component nonresponsive to the antigen. The immunostimulatory class of immunomodulators is more common than the other two types [74]. Although the precise molecular mechanism of herbal immunomodulators is not well understood, they are known to regulate pro-inflammatory and anti-inflammatory cytokines. Another mechanism, such as modulation of the gut microbiome, has also been described [75].
Plant product-derived nanotheranostics for the control of malarial infections
| Plant Product | Importance in Health | Mechanism of Action | Nanotheranostic agent |
|---|---|---|---|
| Plectranthus amboinicus leaf extract | Antimalarial effect | Disruption of epithelial layer and vacuolization of cells of larvae | Zinc oxide nanoparticles [156] |
| Cymbopogon citratus leaf extract | Antimalarial effect | Control of Anopheles and Aedes larval populations | Gold NPs [157] |
| Mimosa pudica Gaertn leaf extract | Antiparasitic activity | Control larvae of plasmodium, Anopheles subpictus Grassi, and Rhipicephalus | Silver NP [158] |
| Curcuminoids | Antiparasitic activity against Plasmodium berghei | Cured infected mice models and showed recrudescence | Liposomes [159] |
| Artemisinin | Parasitemia inhibition | Intravenous administration of artemisinin | Artemisinin nanoparticles [160] |
Plant products demonstrate effectiveness in cancer models
| Plant Products | Cancer Type | Mechanism of Action |
|---|---|---|
| Coffee | Various types of cancer | Induction of cell death, antioxidant and anti-inflammatory effects, DNA damage prevention, antiproliferative activity, angiogenesis inhibition, and suppression of matrix metalloproteases [54] |
| Carotenoids (β-carotene, curcumin, lutein, phytoene, crocin, crocetin, lycopene, β-cryptoxanthin, and astaxanthin) | Breast, ovarian, cervical, and colorectal cancer | Apoptosis induction, gap junction modulation, cell cycle regulation, antiproliferative action, growth factor signaling control, antioxidant response activation, and modulation of differentiation-related proteins [58, 59] |
| Resveratrol | Skin, breast, colon, and liver cancer | Modulating apoptosis through the BAX gene, inhibiting metastasis by repressing the MALAT1 governed EMT [62] |
| EGCG | Prostate, brain, bladder, cervical cancer | Inhibits cell proliferation, extends rat lifespan, upregulates p21Waf1/Cip1, downregulates CDC25A, and induces cancer cell apoptosis via epigenetic regulation of genes like hTERT or by increasing ROS levels [161] |
Plant product-derived nanotheranostics for the control of cancer
| Plant Product | Importance in Health | Mechanism of Action | Nanotheranostic agent |
|---|---|---|---|
| Noscapine | Prostate cancer | Imaging and drug delivery | Iron oxide NPs [162] |
| Resveratrol | Breast cancer | Cytotoxic activity | Polymeric NPs [163] |
| Gelatin | Tongue squamous cell carcinoma cells | Cisplastin loaded CNTs as drug delivery agent | Carbon nanotubes [164] |
| Olive-leaf extracts | Breast, Colorectal, Hepatocellular cancer | Anticancer effect | Multiwalled carbon nanotubes [164] |
| Salvia spinosa extract | Pancreatic cancer | Cytotoxic activity | Graphene oxide [165] |
Several medicinal plants and naturally occurring phytochemicals, such as curcumin, genistein, lectins, indoles, glucans, capsaicin, phytosterols, resveratrol, saponins, tannins, terpenoids, quercetin, polysaccharides, epigallocatechin-3-gallate, flavonoids, isoflavonoids, alkaloids and peptides, colchicine, fatty acids, sesquiterpenes, andrographolide, and labdane diterpenes, have shown to possess immunomodulating properties [74, 76]. Echinacea species, which are widely used in the U.S. for their immunostimulant properties, affect both innate and adaptive immunity. It is known for its antiviral, anti-inflammatory, and antimicrobial effects [74]. It also influences the immune system by modulating the gut microbiota [75]. The immunomodulatory effects of alkylamines and polysaccharides are achieved through enhanced macrophage and lymphocyte activation. Recently, Ligustrum vicaryi L. fruit polysaccharide (LVFP), has been used as an immunomodulator. In an immunocompromised mouse study, LVFP administration increased the spleen and thymus indices. It also enhances neutrophil phagocytic activity, accelerates B and T-lymphocyte activation, and elevates the serum levels of Interleukin-10 (IL-10) and tumor necrosis factor alpha (TNF-α). These findings indicate that LVFP stimulates both innate and adaptive immunity and possesses anti-inflammatory properties [77]. The health benefits of curcumin (present in Curcuma longa L.), including its immunomodulatory properties, are accepted worldwide [77-79]. Immunomodulation is achieved by activating T-cells and other immune cells [80]. A recent study conducted on the Huh-7 cell line indicated that EGYVIR, a mixture of black pepper and curcumin extract, has antiviral activity against SARS-CoV-2. The virus is inactivated by suppressing the NF-kβ pathway, which reduces the release of Interleukin-6 (IL-6) and TNFα [80, 81] The role of turmeric in enhancing the immune system, particularly in respiratory diseases such as COVID-19, has been explored [79]. A study on nanoparticle-mediated delivery of curcumin revealed that curcumin stimulated cell-mediated and humoral immunity in mice [77]. Although plant-based immunomodulators have variable effects, they have immense scope due to their low toxicity and better efficacy when used in combination with other herbal or chemical immunomodulators (Table 7). Drug formulations, through the encapsulation of potential compounds in nanoparticles and their surface modification or capping of nanomaterials, serve as potent immunomodulatory nanotheranostics. Plant-derived immunomodulators serve as nanocarrier-based nanosystems and have been proven to be effective against autoimmune diseases, tumors, inflammation, immune cell function regulation, cytokine neutralization, enzyme-like activity, etc [82]. Various formulations and alterations in administration methods, along with advancements in nanotechnology-based delivery systems, have addressed critical pharmaceutical challenges associated with the pharmacokinetics of curcumin, as listed in Table 8. These efforts aim to enhance its therapeutic efficacy, offering renewed optimism for the clinical application of this natural compound [83].
Immunomodulatory role of plant products
| Plant-Derived Product | Source | Immunomodulatory Effects |
|---|---|---|
| Resveratrol | Grape skin, peanuts, legumes, berries | Stimulates natural defense mechanisms, anti-inflammatory, neuroprotective, anticancer, improves insulin sensitivity, increases longevity [62] |
| Quercetin | Onion, broccoli, herbs, wine, fruits, tea | Inhibits inflammatory mediators, suppresses lipoxygenase enzyme activity, alleviates mast cell membranes, attenuates drug-induced oxidative stress, inhibits mast cell activation-induced release of cytokines [166] |
| Opuntia ficus-indica | Nopal cactus (cladodes, fruits, flowers) | Antioxidant, neuroprotective, hepatoprotective, anticancer, inhibits nitric oxide production, increases antioxidant activity [93] |
| Aqueous extract from tomatoes | Tomatoes | Reduces inflammation in macrophages, alters expression of interleukins and chemokines, improves immune regulation [94] |
| KIOM-MA | Herbal medicine | Inhibitory effect on pro-inflammatory mediators in LPS-stimulated cells, used to treat atopic dermatitis and asthma [95] |
| Potentilla erecta (PE) | Rosaceae family | Inhibits ultraviolet-B (UVB)-induced upregulation of cyclooxygenase-2 (COX-2), reduces level of Prostaglandin E2 (PGE2), exhibits anti-inflammatory role both in vivo and in vitro [96] |
| 17-hydroxy-jolkinolide B derivative (HJB-1) | Unknown source | Attenuates LPS-induced pulmonary histological alteration, lung edema, inflammatory cells infiltration, cytokines, NF-kB, and MAPK activation [97] |
| Koumine | Gelsemium elegans | Blocks ROS production induced by LPS, p53 activation, and mitochondrial dysfunction [98] |
| Ethyl acetate phase (EtOAc) of A. occidentale | A. occidentale L bark | Reduces edema and levels of IL-1β and TNF-α [167] |
Plant product-derived nanotheranostics for immunomodulatory effects
| Plant Product | Importance in Health | Mechanism of Action | Nanotheranostic agent | Reference |
|---|---|---|---|---|
| Curcumin | Immunomodulatory effect with reduced replication of Human Immunodeficiency Virus (HIV) | Antiretroviral agents, inhibiting expression of pro-inflammatory mediators induced by HIV-1 infection | Silver nanoparticles | [83] |
| Curcumin | Modulating vascular deposition of circulating tumor cells | Prevent metastasis, modulate vascular inflammation | Lipid-polymer nanoparticles | [168] |
| Hypoxis hemerocallidea- Hypoxoside | Immunomodulatory effects in macrophages and Natural killer cells (NK) | Lower pro-inflammatory cytokine levels in macrophages. Reduce expression of cytokines in NK cells | Gold NPs | [169] |
Natural product-based nanotheranostics for inflammation
Inflammation is a complex immune response triggered by physical factors, leading to symptoms such as fever and fatigue. It involves the production of proinflammatory cytokines and chemokines, as well as reduced antioxidant defenses. Lipid intermediates from arachidonate metabolism also contribute to inflammation-related diseases such as cancer and cardiovascular issues. Inflammation is linked to various conditions, including cancer, obesity, neurodegenerative diseases, diabetes, and demyelination. Natural compounds with anti-inflammatory properties show promise for preventing or treating these conditions. Resveratrol is a well-studied polyphenolic compound with pleiotropic effects that is commonly found in grape skin, peanuts, legumes, and berries. It stimulates natural defense mechanisms in plants, along with beneficial effects in animals and humans, such as playing important roles in neuroprotection, anticancer activity, improving insulin sensitivity, increasing longevity, and exhibiting anti-inflammatory properties [84-88]. Quercetin, a well-studied polyphenol, and flavonoid are found in onion, broccoli, herbs, wine, fruits, and tea. Quercetin plays a crucial role in preventing inflammation through the inhibition of inflammatory mediators and the suppression of lipoxygenase enzyme activity. Quercetin reduces the synthesis and secretion of inflammatory mediators and histamine, mainly by alleviating the cell membranes of mast cells [89, 90]. Isoniazid and rifampicin are drugs that are routinely used to treat tuberculosis (TB); however, their use can cause severe liver damage, potentially leading to liver failure. Prophylactic quercetin treatment reduced oxidative stress caused by anti-TB drugs by enhancing NRF-2 activation, which in turn lessened the severity of liver inflammation and necrosis [91]. Opuntia ficus-indica, commonly known as Nopal cactus, is used in traditional medicine in sub-Saharan regions and is sourced from various aerial parts, including cladodes, fruits, and flowers, and has antioxidant, flavonoid, neuroprotective, hepatoprotective, and anticancer activities [33, 92]. In addition, extracts derived from Nopal cactus exhibit neuroprotective activity by inhibiting nitric oxide production and increasing antioxidant activity in microglial BV-2 cells [93]. The aqueous extracts from tomatoes reduced inflammation in macrophages. In parallel, the expression of interleukins and chemokines in human peripheral blood leukocytes (PBLs) is altered, suggesting that aqueous tomato extract blunts inflammation and enhances immune regulation [94]. An herbal medicine, KIOM-MA, which is used to treat atopic dermatitis and asthma, also suppressed proinflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 cells by attenuating the LPS-induced increases in nitric oxide synthase, COX-2, nitric oxide, and PGE2. PGE2 were blocked by the administration of KIOM-MA in vitro [95]. Potentilla erecta (PE) belongs to the Rosaceae family and is traditionally used to treat diarrhea, hemostasis, and hemorrhoids. PE inhibited UVB-induced upregulation of COX-2 and dose-dependently reduced the level of PGE2. These findings suggest that PE fractions containing high amounts of agrimoniin play an anti-inflammatory role in vitro and in vivo [96]. A derivative of 17-hydroxy-jolkinolide B (HJB-1) exhibited an anti-inflammatory effect against LPS-induced acute respiratory distress syndrome (ARDS) in vitro. HJB-1 markedly reduced LPS-induced pulmonary histological changes, lung edema, inflammatory cell infiltration, as well as cytokine, NF-kB, and MAPK activation [97]. Koumine is an alkaloid extracted from Gelsemium elegans that blocks LPS-induced ROS production, p53 activation, and mitochondrial dysfunction in RAW 264.7 macrophages, suggesting that koumine potentially has a protective effect against LPS-induced damage [98]. The bark of A. occidentale L. demonstrates various biological activities, including antioxidant, antimicrobial, and anti-inflammatory effects. The ethyl acetate extract (EtOAc) of A. occidentale was found to reduce edema and lower levels of IL-1β and TNF-α, indicating that EtOAc plays a critical role in modulating the inflammatory response in a preclinical model [99-101]. NPs and nano-emulsions are employed to target inflammation by recognizing molecules expressed on the surface of inflammatory cytokines or endothelial cells, taking advantage of increased vascular permeability, or using biomimicry [102]. These strategies offer promising approaches for treating inflammatory diseases. The widespread use of natural compounds for preventing and treating various diseases is attributed to their antioxidative and anti-inflammatory properties. As promising natural compounds, plant-derived exosome-like nanoparticles (PELNs) are derived from the multivesicular bodies of various edible plants, including vegetables, foods, and fruits. These nanoparticles can effectively restore the balance between proinflammatory and anti-inflammatory effects in a range of diseases, including colitis, cancer, and inflammation-related metabolic disorders [103]. Recent scientific interest has focused on the use of extracellular vesicles (EVs) derived from natural compounds as potential treatments for inflammatory diseases. These nanometer sized EVs, which are isolated from plants such as grapefruit and Dendropanax mellifera, are efficiently taken up by host organs and can influence both physiological and pathological processes [104]. Additionally, nanoparticles made from albumin and cerium oxide, synthesized through biomineralization, exhibit enzymatic-like properties and can scavenge ROS. These nanoparticles reprogram macrophages from a proinflammatory state to an anti-inflammatory state. In the collagen-induced arthritis (CIA) mouse model, the nanoparticles accumulated in inflamed joints and demonstrated therapeutic efficacy comparable to that of established rheumatoid arthritis treatments [105]. Table 9 summarizes some studies pertaining to plant-based nanotheranostics for inflammatory disorders.
Natural product-based nanotheranostics for hepatoprotective properties
Natural bioactive components sourced from plant secondary metabolites are increasingly recognized as valuable alternatives for preventing and alleviating hepatotoxic effects and their chronic complications. They possess antifibrotic, antioxidant and antihepatotoxic properties. This review delves into the mechanisms of action of promising plant-derived products for treating patients with various liver disease [106]. The incorporation of nanotheranostic agents such as nanoparticles and carbon-based nanoderivatives further improves the theranostic efficiency of beneficial hepatoprotective regimens. The hepatoprotective activity of silver NPs has been demonstrated using a plant extract of Rhizophora apiculata as a reducing agent to prepare NPs. This study incorporated a mouse model induced with carbon tetrachloride, and the activity of R. apiculata with silver NPs was studied. As a result, the nanoparticles exhibited hepatoprotective effects that were derived from mangrove ecosystem plant extracts [107]. Another report by Li et al. described a silibinin-loaded hyaluronic acid (HA) micelle. This micelle binds specifically to CD44 receptors, which are abundant on the membrane of activated hepatic stellate cells (aHSCs). These micelles exhibited exceptional targeting capabilities toward fibrotic liver tissues and selectively bound further, eliminating aHSCs [108]. Consequently, this approach has demonstrated a highly effective anti-hepatic fibrosis effect. Various natural products for combined components of hepatoprotective disorders are tabulated below in Table 10.
Nonalcoholic fatty liver disease (NAFLD)
Nonalcoholic fatty liver disease (NAFLD) is a chronic liver condition with limited treatment options. Clinically, it encompasses a wide range of hepatic anomalies, that includes nonalcoholic steatohepatitis (NASH), simple steatosis, fibrosis, and cirrhosis [109]. The accumulation of intolerant levels of fats in the liver (other than alcohol abuse) is characteristic of NAFLD [110]. NAFLD is reportedly associated with other metabolic disorders, such as type II diabetes, hyperlipidemia, obesity, CVD, and chronic kidney disease (CKD) [111-113]. In the recent past, the incidence of NAFLD has increased drastically due to dietary changes and urbanized lifestyles [110, 114]. The results of several studies suggest that natural bioactive components of herbs and the diet can be studied for the prevention and treatment of NAFLD. The Qushi Huayu Fang (QHF) mixture of five herbal medicines, Polygonum cuspidatum, Curcuma longa, Artemisia capillaris, Gardenia jasminoides, and Hypericum japonicum, is traditionally used in China for the treatment of NAFLD [115]. In a study on NAFLD rats, QHF administration decreased the deposition of fat in liver and recovered liver pathology through reduced fat degeneration, hepatocyte ballooning, and inflammation [112]. Several other mechanisms for the anti-NAFLD activity of QHF are also discussed, including the upregulation of peroxisomal proliferator-activated receptor α (PPARα), regulation of adipocytokine release, the suppression of insulin signaling, the downregulation of Sterol Regulatory Element Binding Protein-1c (SREBP-1c) levels, the suppression of systemic and macrophage inflammation, and the modulation of the gut microbiome [115] In China, for several years, Fufang-Zhenzhu-Tiao Zhi Capsule (FTZ), a cocktail of eight Chinese medical herbs, improved NAFLD in mice by altering the gut microbiota. An increased Bacteroidetes ratio and a decreased Firmicutes/Bacteroidetes ratio are correlated with reduced lipogenic gene expression, including genes such as SCD1, FAS, CD36, and C/EBP-α [116]. Phloroglucinol (PHG), a phenolic compound found in some plants, is used to treat gastric disorders. A study on HepG2 cells exposed to hydrogen peroxide or palmitic acid revealed that PHG has strong antioxidant properties that are comparable to those of α-lipoic acid (ALA) and N-acetylcysteine (NAC). PHG also reduces inflammation and apoptosis in treated cells [117]. Another study demonstrated the therapeutic effect of vine tea polyphenol (VTP), a Chinese herb derived from Ampelopsis grossedentata, in WD-induced NAFLD mice (C57BL/6 N). These results suggest that dihydromyricetin (a bioactive component of VTP) reverses the effects of WD by decreasing the serum triglyceride and cholesterol levels, decreasing the accumulation of lipids in the liver, and modulating the gut microbiota [118]. Recent advancements in the use of polymeric nanoparticles such as nanocurcumin have led to the investigation of diverse techniques to address hepatic disorders. For example, the use of polylactic-co-glycolic acid (PLGA) nanoparticles has resulted in a 22-fold increase in curcumin bioavailability. Curcumin has the potential to enhance the onset and progression of NAFLD by effectively inhibiting inflammation and oxidative stress [119].
Alcoholic Liver Disease (ALD)
Chronic alcohol consumption causes liver damage and is considered as one of the most common causes of chronic liver disease [120]. According to the 2018 WHO report, 3 million deaths are reported every year due to alcohol abuse, accounting for 5.3% of the total deaths worldwide. ALD causes several changes in the liver, from simple steatosis to cirrhosis and acute alcoholic hepatitis [121]. The global burden of ALD is increasing, yet treatment options remain limited. The clinical presentation of ALD varies among individuals and changes over time. Herbal products, with their multiple targets and fewer side effects, offer potential for preventing cirrhosis and improving patient life expectancy [122, 123]. Silybum marianum derived flavonoid, Silymarin, is widely used in the treatment of liver disease. A recent meta-analysis revealed that it acts as an antioxidant, reduce ROS production and lipid peroxidation and thereby protects cells.
Plant product-derived nanotheranostics for inflammatory disorders
| Plant Product | Importance in Health | Mechanism of Action | Nanotheranostic agent |
|---|---|---|---|
| Anoectochilus elatus Leaf Extract | Anti-inflammatory | inhibition of pro-inflammatory cytokines and suppression of NF-κB signaling pathways. | Silver nanoparticles [105] |
| Ginger | Ulcerative colitis | reduced expression of CD98 | Ginger-derived lipid nanoparticles [170] |
| Puerarin, a flavonoid from Pueraria lobata | Anti-inflammatory | M1 macrophages polarization to M2 | Exosome-like nanoparticles [171] |
| Blueberry | Vascular system | Regulate the expression of inflammatory genes in response to TNF-α | Exosome-like nanoparticles [172] |
| Panax ginseng leaves | Anti-inflammatory | Blockade of NF-κB activation in macrophages | Gold NPs [173] |
Plant product-derived nanotheranostics for hepatoprotective disorders
| Plant Product | Importance in Health | Mechanism of Action | Nanotheranostic agent |
|---|---|---|---|
| Curcumin | Hepatic cancer | Drug delivery system for hepatoma cells | Lactosylated curdlan-triornithine nanocarriers [174] |
| Blueberry | NAFLD | Reduce oxidative stress, reactive oxygen species, prevented cell apoptosis in hepatic cells | blueberry-derived exosomes-like nanoparticles [175] |
| Celastrol | NAFLD | Hepatoprotective activity | Albumin-based nanoparticles [176] |
| Chitosan and Silymarin | NAFLD | Protective effect against liver steatosis | Polymer hybrid nanoparticles [177] |
| Nicotinamide and ascorbic acid nanoparticles | NAFLD | Decreased oxidative and nitrosative stresses | Chitosan nanoparticles [178] |
| Glycyrrhizin | Hepatocyte drug delivery | Decline of the tissue damage | Chitosan nanoparticles [179] |
| Ginger | ALD | Inhibited reactive oxygen species production | Ginger extracts nanoparticles [180] |
| Quercetin | Liver cancer | Inactivation of signaling pathways causing cancer | Gold-quercetin nanoparticles [180] |
| Quercetin | ALD | activates autophagy and restore lysozyme function by combating exosomes release | Quercetin exosomes [181] |
| Silymarin and quercetin | NAFLD | hepatoprotective activity | Miniaturized scaffold [182] |
| Silymarin | ALD | Inversion of biochemical parameters and histopathological alterations | Silymarin Phytosome nanoparticles [183] |
| Glycyrrhiza glabra (Licorice) root extract | Liver disease | Anti-Inflammatory effect in liver diseases | Nanoparticles [184, 185] |
This study also revealed that oral silymarin lowers serum alanine aminotransferase (ALT) and aspartate Aminotransferase (AST) levels, although the reduction was not clinically significant, warranting further research. Its anti-inflammatory effects are achieved by inhibiting NF-κB, which reduces the levels of inflammatory cytokines in liver cells [124]. In an ethanol-induced mouse model, Pueraria lobata and Silybum marianum showed greater efficacy in reducing steatosis and hepatic inflammation when used together than when used alone. This effect was linked to enhanced LKB1/AMPK/ACC signaling and reduced LPS-triggered TLR4-mediated NF-κB signaling [125]. Garlic (Allium sativum L. [Amaryllidaceae]) is traditionally used to treat liver disease [126]. Garlic polysaccharide (GP) has a significant hepatoprotective effect on ALDmodel mice. GP treatment reduced oxidative stress and lipid peroxidation and modified three pathways, namely, TNF-α, TGF-β1 and decorin pathways, to prevent hepatic stellate cell (HSC) activation and reduce the production of the extracellular matrix (ECM). Further modulation of the gut microbiota by GP treatment has also been reported [127]. Glycyrrhizin, obtained from the roots of Glycyrrhiza glabra (licorice), exhibits potent hepatoprotective properties. In one study, its impact on alcohol consumption was evaluated through a randomized, placebo-controlled, double-blind, trial involving 12 healthy participants (six males and six females). The results suggest that, compared with alcohol intake without glycyrrhizin, the intake of alcohol with glycyrrhizin improves liver function [128]. In another study, mice were given alcohol with or without licorice. The negative effects of alcohol consumption were reversed in the mice treated with licorice, a result attributed to its antioxidant and anti-inflammatory properties [129]. Conventional diagnostics and drug treatments often lack precision and effectiveness. Nanomedicine, a promising field in medical nanotechnology, offers improved imaging, enhanced tissue penetration, and targeted drug delivery for alcoholic liver disease. It also combines diagnosis and therapy, functioning as a nanotheranostic [130]. The hepatoprotective effect of ginger against certain types of toxicity has been studied extensively. Shogaols, which are dehydrated gingerol analogs, are a major focus of research pertaining to anti-inflammatory effects in the liver [131]. Quercetin is also used in nanotheranostics, as demonstrated by a study that developed a more biologically available liposomal formulation to assess its effects on liver damage in male rats. Researchers investigated the effects of nanoliposome-delivered quercetin on liver damage induced by amoxicillin/clavulanate, focusing on its impact on the NF-κB/SIRT1/Nrf2 signaling pathway and microbiota modulation. The study found reduced hepatic damage, as indicated by improved serum liver enzymes, enhanced antioxidant status, and changes in microbiota [132].
Viral hepatitis
Viral hepatitis is a major health issue representing a systemic infection that affects mainly the hepatic system [133-135]. Five classes of viruses have been identified and named hepatitis A, B, C, D, and E. All five strains affect liver function but differ in their structure, transmission mode, lethality, preventive technique, and geographical spread. At present, no vaccines are available for all classes of viral infection. Owing to the differences mentioned above, the vaccine developed for one type of virus cannot be 100% effective against other classes. Additionally, due to the adverse effects and high cost of the vaccine, attention to alternative treatment strategies, such as traditional herbs, has been explored to identify a cure for viral infection. Glycyrrhizic acid (GA), a bioactive component of liquorice, has been shown to improve hepatic inflammation (murine hepatitis virus (MHV)-A59-induced model) by modulating the HMGB1-TLR4 signaling pathway. The protective effect of GA treatment was due to reduced IL-17 and IL-22 levels [136]. A study highlighted the importance of several Indian medicinal plants, including Phalatrikadi Kwath, Triphala, Vasa (Adhatoda vasica), Giloy (Tinospora cordifolia), Neem (Azadirachta indica), Kiratatikta (Swertia chirayita), Katuki (Picrorhiza kurroa), Kalmegh (Andrographis paniculata), Musta and Nagaramustaka (Cyperus rotundus and Cyperus eleusinoides), and Bhumyamalaki (Phyllanthus niruri), for their hepatoprotective role against hepatitis infection. The significant advantages of nanoparticle-based drug delivery systems have paved the way for developing strategies targeting the hepatitis B virus (HBV) [137]. There has been a notable increase in various nanoparticle formulations for anti-HBV therapy, integrating features from different delivery systems used in anti-HBV approaches such as vaccines, gene therapy, and nucleoside drugs [9]. Various nanotheranostic agents, such as lipid molecules and polymeric micelles, have been effectively employed for developing drug delivery systems for anti-HBV-based gene therapy, and RNA interference (RNAi) therapeutics have been used for antiviral treatment of plant-based products [138]. From this perspective, herbal polymer-based nanomedicines can be easily tailored for optimal physicochemical attributes and functions, offering significant advantages in treating viral hepatitis. Compared with conventional therapeutic agents, they contribute to improved therapeutic outcomes and mitigate adverse drug effects [139]. Various studies have reported that plant-derived nanovesicles (PNVs), which are natural disease therapeutics, are eco-friendly, have low toxicity, high yield, simplicity, safety, etc., and have a low risk of in vivo immunogenicity [140]. There are certain examples listed in the literature in Table 11 consisting of various plant-based products for nanotheranostics in viral hepatitis.
Plant product-derived nanotheranostics for viral hepatitis
| Plant Product | Importance in Health | Mechanism of Action | Nanotheranostic agent |
|---|---|---|---|
| Mannose | Hepatitis B vaccine delivery | Hepatitis B surface antigen loaded inside MNP strengthened humoral and cellular immune responses | Mannose-modified poly D, L-lactide-co-glycolic acid (PLGA) nanoparticles (MNP) [186] |
| Ferritin | Chronic hepatitis B | Ferritin NPs delivering preS1 as vaccine candidate including SIGNR1+ dendritic cells | Ferritin nanoparticle [187] |
| Stearic acid and Chitosan | Hepatitis B virus | Inhibition of HBeAg expression by complex micelle and 10-23 DNAzyme | Complex Micelle [188] |
| Chitosan | Hepatitis B Virus | Inhibitory effects on antigen expression and DNA replication | Chitosan micelles containing Lamivudine stearate [189] |
Summary
The literature suggests that natural plant products exhibit significant biological and pharmacological activities. Although plant-based medicines have been used for centuries, their effectiveness is often limited by poor bioavailability, low stability, and nonspecific targeting. Recent advancements in nanotechnology have introduced the concept of "nanotheranostics," which combines therapeutic and diagnostic functions within a single nanostructure. By incorporating plant-based compounds into nanoparticles, researchers have developed innovative nanotheranostic agents. These agents significantly enhance drug delivery, targeted therapy, and disease diagnosis. These nanotheranostic agents work by encapsulating plant-derived active ingredients within nanoparticles, which are engineered to target specific cells or tissues. The nanoparticles can be designed to release the drug in a controlled manner, ensuring that the therapeutic compounds reach the diseased site with minimal side effects on healthy tissues. Additionally, these nanostructures can be equipped with imaging agents that allow real-time monitoring of drug delivery and disease progression through various imaging techniques, such as MRI, PET, or fluorescence imaging. The integration of plant-based medicines with nanotechnology offers a promising approach for more effective and personalized treatments. Nanotheranostic not only improve the therapeutic efficacy of plant-derived drugs but also provide tools for early and accurate disease diagnosis, making them a powerful asset in modern medicine. Important benefits of nanotheranostic agents are also illustrated in Fig. 3.
Future Directions
The integration of plant-derived natural products with nanotechnology holds immense potential for advancing healthcare. Future research should focus on optimizing the synthesis and functionalization of nanotheranostic agents to enhance their biocompatibility, targeting efficiency, and therapeutic efficacy. The development of multifunctional nanoparticles that can simultaneously diagnose, deliver drugs, and monitor treatment response in real-time will revolutionize personalized medicine. Additionally, exploring the use of plant-based exosomes and other nanovesicles as delivery systems could open new avenues for non-invasive diagnostics and targeted therapies. Collaborative efforts between multidisciplinary teams, including chemists, biologists, and clinicians, will be crucial in translating these innovations from the laboratory to clinical practice. By harnessing the unique properties of natural products and advanced nanomaterials, we can pave the way for more effective, safe, and personalized treatment strategies for a wide range of diseases.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Shiva MP. Inventory of Forest Resources for Sustainable Management & Biodiversity Conservation with Lists of Multipurpose Tree Species Yielding Both Timber & Non-timber Forest Products (NTFPs), and Shrub & Herb Species of NTFP Importance: Indus Publishing Company; 1998
2. Kala CP. Status and conservation of rare and endangered medicinal plants in the Indian trans-Himalaya. Biological Conservation. 2000;93(3):371-9
3. Rao MR, Palada MC, Becker BN. et al. Medicinal and aromatic plants in agroforestry systems. Agroforestry Systems. 2004
4. Kinghorn AD. Pharmacognosy in the 21st century*. Journal of Pharmacy and Pharmacology. 2001 53(2)
5. Khan N. Nanotheranostics-An Emerging Technique in Nanomedicine. American Journal of Biomedical Science & Research. 2021;14:35-9
6. Muddapur UM, Alshehri S, Ghoneim MM, Mahnashi MH, Alshahrani MA, Khan AA. et al. Plant-Based Synthesis of Gold Nanoparticles and Theranostic Applications: A Review. Molecules [Internet]. 2022 27(4)
7. Gupta A, Koirala AR, Gupta B, Parajuli N. Improved method for separation of silver nanoparticles synthesized using the Nyctanthes arbor-tristis shrub. Acta Chemica Malaysia. 2019;3(1):35-42
8. Dewi MK, Chaerunisaa AY, Muhaimin M, Joni IM. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials (Basel). 2022 12(22)
9. Miao T, Floreani R, Liu G, Chen X. Nanotheranostics-Based Imaging for Cancer Treatment Monitoring: Advanced Materials, Methods, and Devices. 2019. p. 395-428.
10. Sneider A, VanDyke D, Paliwal S, Rai P. Remotely Triggered Nano-Theranostics For Cancer Applications. Nanotheranostics. 2017;1(1):1-22
11. Silva CO, Pinho JO, Lopes JM, Almeida AJ, Gaspar MM, Reis C. Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics. 2019 11(1)
12. Scheinberg DA, Villa CH, Escorcia FE, McDevitt MR. Conscripts of the infinite armada: systemic cancer therapy using nanomaterials. Nat Rev Clin Oncol. 2010;7(5):266-76
13. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF. et al. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 2016;1(5):16014
14. Chaachouay N, Azeroual, A, & Zidane, L.(Eds.). Comprehensive Guide to Hallucinogenic Plants. CRC Press. (2025).
15. Kim SH, Jo SH, Kwon YI, Hwang JK. Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. Int J Mol Sci. 2011;12(6):3757-69
16. Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001 56(9)
17. Kumari K, Augusti KT. Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. Journal of Ethnopharmacology. 2007 109(3)
18. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comparative Biochemistry and Physiology - C Toxicology and Pharmacology. 2003 135(3)
19. Kang YR, Choi HY, Lee JY, Jang SI, Kang H, Oh JB. et al. Calorie Restriction Effect of Heat-Processed Onion Extract (ONI) Using In vitro and In vivo Animal Models. Int J Mol Sci. 2018 19(3)
20. Nagappan A, Jung DY, Kim JH, Jung MH. Protective effects of gomisin n against hepatic cannabinoid type 1 receptor-induced insulin resistance and gluconeogenesis. International Journal of Molecular Sciences. 2018 19(4)
21. Pathak K, Saikia R, Sarma H, Pathak MP, Das RJ, Gogoi U. et al. Nanotheranostics: application of nanosensors in diabetes management. J Diabetes Metab Disord. 2023;22(1):119-33
22. Krol S, Ellis-Behnke R, Marchetti P. Nanomedicine for treatment of diabetes in an aging population: state-of-the-art and future developments. Nanomedicine. 2012;8(Suppl 1):S69-76
23. Yang X, Lee HM, Chan JC. Drug-subphenotype interactions for cancer in type 2 diabetes mellitus. Nat Rev Endocrinol. 2015;11(6):372-9
24. Dini S, Zakeri M, Ebrahimpour S, Dehghanian F, Esmaeili A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles modulate glucose metabolism-related genes and miR-29 family in the hippocampus of diabetic rats. Sci Rep. 2021;11(1):8618
25. Dewanjee S, Chakraborty P, Mukherjee B, De Feo V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int J Mol Sci. 2020 21(6)
26. Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harbor Perspectives in Biology. 2018 10(4)
27. Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. Journal of Cell Biology2009.
28. Zeng XS, Geng WS, Jia JJ, Chen L, Zhang PP. Cellular and molecular basis of neurodegeneration in Parkinson disease. Frontiers in Aging Neuroscience2018.
29. Taylor JP, Brown RH, Cleveland DW. Decoding ALS: From genes to mechanism. Nature2016.
30. Sabti M, Sasaki K, Gadhi C, Isoda H. Elucidation of the molecular mechanism underlying lippia citriodora(Lim.)-Induced relaxation and Anti-Depression. International Journal of Molecular Sciences. 2019;20(14)
31. Lee JC, Kim HR, Kim J, Jang YS. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. Journal of Agricultural and Food Chemistry. 2002 50(22)
32. Jang Y, Choo H, Lee MJ, Han J, Kim SJ, Ju X. et al. Auraptene mitigates parkinson's disease-like behavior by protecting inhibition of mitochondrial respiration and scavenging reactive oxygen species. International Journal of Molecular Sciences. 2019 20(14)
33. Bhattacharyya S, Bhattacharya R, Curley S, McNiven MA, Mukherjee P. Nanoconjugation modulates the trafficking and mechanism of antibody induced receptor endocytosis. Proceedings of the National Academy of Sciences. 2010;107(33):14541-6
34. Burns J, Buck AC, S DS, Dube A, Bardien S. Nanophytomedicines as Therapeutic Agents for Parkinson's Disease. ACS Omega. 2023;8(45):42045-61
35. Menaa F, Wijesinghe U, Thiripuranathar G, Althobaiti NA, Albalawi AE, Khan BA. et al. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar Drugs. 2021 19(9)
36. Kundu P, Das M, Tripathy K, Sahoo SK. Delivery of Dual Drug Loaded Lipid Based Nanoparticles across the Blood-Brain Barrier Impart Enhanced Neuroprotection in a Rotenone Induced Mouse Model of Parkinson's Disease. ACS Chem Neurosci. 2016;7(12):1658-70
37. Ashfaq R, Rasul A, Asghar S, Kovács A, Berkó S, Budai-Szűcs M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int J Mol Sci. 2023 24(21)
38. Mojab F. Antimalarial natural products: a review. Avicenna journal of phytomedicine. 2012 2(2)
39. Bekono BD, Ntie-Kang F, Onguéné PA, Lifongo LL, Sippl W, Fester K. et al. The potential of anti-malarial compounds derived from African medicinal plants: A review of pharmacological evaluations from 2013 to 2019. Malaria Journal2020.
40. Uzor PF. Alkaloids from Plants with Antimalarial Activity: A Review of Recent Studies. Evidence-based Complementary and Alternative Medicine2020.
41. Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN. et al. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malaria Journal2011.
42. Xia M, Liu D, Liu Y, Liu H. The Therapeutic Effect of Artemisinin and Its Derivatives in Kidney Disease. Frontiers in Pharmacology2020.
43. Xia M, Liu D, Liu Y, Liu H. The Therapeutic Effect of Artemisinin and Its Derivatives in Kidney Disease. Front Pharmacol. 2020;11:380
44. Diallo MA, Yade MS, Ndiaye YD, Diallo I, Diongue K, Sy SA. et al. Efficacy and safety of artemisinin-based combination therapy and the implications of Pfkelch13 and Pfcoronin molecular markers in treatment failure in Senegal. Scientific Reports. 2020 10(1)
45. van der Pluijm RW, Amaratunga C, Dhorda M, Dondorp AM. Triple Artemisinin-Based Combination Therapies for Malaria - A New Paradigm? Trends in Parasitology2021.
46. Shankar R, Deb S, Sharma BK. Antimalarial plants of northeast India: An overview. J Ayurveda Integr Med. 2012;3(1):10-6
47. Asadollahi A, Khoobdel M, Zahraei-Ramazani A, Azarmi S, Mosawi SH. Effectiveness of plant-based repellents against different Anopheles species: A systematic review. Malaria Journal2019.
48. Maia MF, Kliner M, Richardson M, Lengeler C, Moore SJ. Mosquito repellents for malaria prevention. Cochrane Database of Systematic Reviews2018.
49. Oga EF, Singh KK. Exploring Nanotechnologies for the Effective Therapy of Malaria Using Plant-Based Medicines. Curr Pharm Des. 2016;22(27):4232-46
50. Rathod R, Pathak B, et al. Green Synthesis of Nanoparticles by Mangrove Plants and Its Biomedical Application2020
51. Velayutham K, Ramanibai R. Larvicidal activity of synthesized silver nanoparticles using isoamyl acetate identified in Annona squamosa leaves against Aedes aegypti and Culex quinquefasciatus. The Journal of Basic & Applied Zoology. 2016;74:16-22
52. Suman TY, Ravindranath RRS, Elumalai D, Kaleena PK, Ramkumar R, Perumal P. et al. Larvicidal activity of titanium dioxide nanoparticles synthesized using Morinda citrifolia root extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus and its other effect on non-target fish. Asian Pacific Journal of Tropical Disease. 2015;5(3):224-30
53. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians. 2021 71(1)
54. Ismail T, Donati-Zeppa S, Akhtar S, Turrini E, Layla A, Sestili P. et al. Coffee in cancer chemoprevention: an updated review. Expert Opinion on Drug Metabolism and Toxicology2021.
55. Steward WP, Brown K. Cancer chemoprevention: A rapidly evolving field. British Journal of Cancer2013.
56. Ramawat KG, Goyal S. Natural Products in Cancer Chemoprevention and Chemotherapy. Herbal Drugs: Ethnomedicine to Modern Medicine2008.
57. Sehrawat A, Roy R, Pore SK, Hahm ER, Samanta SK, Singh KB. et al. Mitochondrial dysfunction in cancer chemoprevention by phytochemicals from dietary and medicinal plants. Seminars in Cancer Biology2017.
58. Milani A, Basirnejad M, Shahbazi S, Bolhassani A. Carotenoids: biochemistry, pharmacology and treatment. British Journal of Pharmacology2017.
59. Zare M, Norouzi Roshan Z, Assadpour E, Jafari SM. Improving the cancer prevention/treatment role of carotenoids through various nano-delivery systems. Critical Reviews in Food Science and Nutrition2021.
60. Ashrafizadeh M, Rafiei H, Mohammadinejad R, Farkhondeh T, Samarghandian S. Anti-tumor activity of resveratrol against gastric cancer: a review of recent advances with an emphasis on molecular pathways. Cancer Cell International2021.
61. Huang L, Zhang S, Zhou J, Li X. Effect of resveratrol on drug resistance in colon cancer chemotherapy. RSC Advances. 2019 9(5)
62. Yang Z, Xie Q, Chen Z, Ni H, Xia L, Zhao Q. et al. Resveratrol suppresses the invasion and migration of human gastric cancer cells via inhibition of MALAT1-mediated epithelial-to-mesenchymal transition. Experimental and Therapeutic Medicine. 2018
63. Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH. et al. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12(10):1281-305
64. Juneja LR, Kapoor MP, Okubo T, Rao T. (Eds.). Green Tea Polyphenols: Nutraceuticals of Modern Life (1st ed.). CRC Press2013
65. Tang Y, Cao JI, Cai Z, An H, Li Y, Peng YAN. et al. Epigallocatechin gallate induces chemopreventive effects on rats with diethylnitrosamine-induced liver cancer via inhibition of cell division cycle 25A. Molecular Medicine Reports. 2020 22(5)
66. Mokbel K, Wazir U, Mokbel K. Chemoprevention of prostate cancer by natural agents: Evidence from molecular and epidemiological studies. Anticancer Research2019.
67. Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Letters2018.
68. Kumar A, Parmar A, Singh R, Dhiman S. Nanoscience: an overview about nanotheranostics for cancer treatment. Egyptian Journal of Basic and Applied Sciences. 2024;11(1):55-68
69. Vasimalai N, Vilas-Boas V, Gallo J, Cerqueira MF, Menéndez-Miranda M, Costa-Fernández JM. et al. Green synthesis of fluorescent carbon dots from spices for in vitro imaging and tumour cell growth inhibition. Beilstein J Nanotechnol. 2018;9:530-44
70. Naik VM, Bhosale SV, Kolekar GB. A brief review on the synthesis, characterisation and analytical applications of nitrogen doped carbon dots. Analytical Methods. 2022;14(9):877-91
71. Li C-L, Ou C-M, Huang C-C, Wu W-C, Chen Y-P, Lin T-E. et al. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. Journal of Materials Chemistry B. 2014;2(28):4564-71
72. Wang SC, Chen MH. Herbal Medicine for Rare Diseases: Alleviating Symptoms by GMP Herbal Formulations (1st ed.). CRC Press2025
73. Mostafavi E, Iravani S, Varma RS, Khatami M, Rahbarizadeh F. Eco-friendly synthesis of carbon nanotubes and their cancer theranostic applications. Mater Adv. 2022;3(12):4765-82
74. Di Sotto A, Vitalone A, Di Giacomo S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines (Basel). 2020 8(3)
75. Ritchie MR, Gertsch J, Klein P, Schoop R. Effects of Echinaforce(R) treatment on ex vivo-stimulated blood cells. Phytomedicine. 2011;18(10):826-31
76. Jantan I, Ahmad W, Bukhari SNA. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Frontiers in Plant Science. 2015 6
77. Afolayan FID, Erinwusi B, Oyeyemi OT. Immunomodulatory activity of curcumin-entrapped poly d,l -lactic- co -glycolic acid nanoparticles in mice. Integrative Medicine Research. 2018 7(2)
78. Hewlings SJ, Kalman DS. Curcumin: A Review of Its Effects on Human Health. Foods. 2017 6(10)
79. Shankhdhar PK, Mishra P, Kannojia P, Joshi H. Turmeric: Plant immunobooster against covid-19. Research Journal of Pharmacognosy and Phytochemistry. 2020 12(3)
80. Roshdy WH, Rashed HA, Kandeil A, Mostafa A, Moatasim Y, Kutkat O. et al. EGYVIR: An immunomodulatory herbal extract with potent antiviral activity against SARS-CoV-2. PLoS ONE. 2020 15(11 November)
81. Singh V, Kumar A, Sood P. (Eds.). Neuro-Nutraceuticals and Drug Discovery and Delivery in Alzheimer's Disease: Volume 2: Strategies to Unlock the Brain's Fortress (1st ed.). Apple Academic Press2025
82. Dai H, Fan Q, Wang C. Recent applications of immunomodulatory biomaterials for disease immunotherapy. Exploration (Beijing). 2022;2(6):20210157
83. Catanzaro M, Corsini E, Rosini M, Racchi M, Lanni C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules. 2018 23(11)
84. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nature Reviews Drug Discovery2006.
85. Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J. et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. British Journal of Nutrition. 2011 106(3)
86. Colin D, Gimazane A, Lizard G, Izard JC, Solary E, Latruffe N. et al. Effects of resveratrol analogs on cell cycle progression, cell cycle associated proteins and 5fluoro-uracil sensitivity in human derived colon cancer cells. Int J Cancer. 2009;124(12):2780-8
87. Lançon A, Frazzi R, Latruffe N. Anti-oxidant, anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases. Molecules2016.
88. Richard T, Pawlus AD, Iglésias ML, Pedrot E, Waffo-Teguo P, Mérillon JM. et al. Neuroprotective properties of resveratrol and derivatives. Annals of the New York Academy of Sciences. 2011 1215(1)
89. Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflammation and Allergy - Drug Targets. 2010 9(4)
90. Finn DF, Walsh JJ. Twenty-first century mast cell stabilizers. British Journal of Pharmacology2013.
91. Sanjay S, Girish C, Toi PC, Bobby Z. Quercetin Modulates Nrf2 and NF-κB/TLR-4 Pathways to Protect against Isoniazid and Rifampicin Induced Hepatotoxicity <i>in vivo</i>. Canadian Journal of Physiology and Pharmacology. 2021
92. El-Mostafa K, El Kharrassi Y, Badreddine A, Andreoletti P, Vamecq J, El Kebbaj MhS. et al. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules2014.
93. Saih FE, Andreoletti P, Mandard S, Latruffe N, El Kebbaj MHS, Lizard G. et al. Protective effect of cactus cladode extracts on peroxisomal functions in microglial BV-2 cells activated by different lipopolysaccharides. Molecules. 2017 22(1)
94. Schwager J, Richard N, Mussler B, Raederstorff D. Tomato aqueous extract modulates the inflammatory profile of immune cells and endothelial cells. Molecules. 2016 21(2)
95. Oh YC, Cho WK, Jeong YH, Im GY, Kim A, Hwang YH, et al. A novel herbal medicine KIOM-MA exerts an anti-inflammatory effect in LPS-stimulated RAW 264.7 macrophage cells. Evidence-based Complementary and Alternative Medicine. 2012;2012
96. Hoffmann J, Casetti F, Bullerkotte U, Haarhaus B, Vagedes J, Schempp CM. et al. Anti-inflammatory effects of agrimoniin-enriched fractions of Potentilla erecta. Molecules. 2016 21(6)
97. Xu X, Liu N, Zhang YX, Cao J, Wu D, Peng Q. et al. The protective effects of HJB-1, a derivative of 17-Hydroxy-Jolkinolide B, on LPS-Induced Acute distress respiratory syndrome mice. Molecules. 2016 21(1)
98. Yuan ZH, Liang ZE, Wu J, Yi JE, Chen XJ, Sun ZL. A potential mechanism for the anti-Apoptotic property of koumine involving mitochondrial pathway in LPS-Mediated RAW264.7 Macrophages. Molecules. 2016;21(10)
99. Vilar MS, de Souza GL, Vilar Dde A, Leite JA, Raffin FN, Barbosa-Filho JM. et al. Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of Anacardium occidentale L. Bark. Molecules. 2016 21(8)
100. Ali S, Friedman SL, Mann DA. Liver diseases: biochemical mechanisms and new therapeutic insights. V. 1. Enfield, NH: Science Publishers. 2006
101. Wang M, Zhao J, Chen J, Long T, Xu M, Luo T. et al. The role of sirtuin1 in liver injury: molecular mechanisms and novel therapeutic target. PeerJ. 2024;12:e17094
102. Puja AM, Rupa EJ, Kim YJ, Yang D-C. Medicinal Plant Enriched Metal Nanoparticles and Nanoemulsion for Inflammation Treatment: A Narrative Review on Current Status and Future Perspective. Immuno. 2023;3(2):182-94
103. Yi Q, Xu Z, Thakur A, Zhang K, Liang Q, Liu Y. et al. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol Res. 2023;190:106733
104. Xu F, Valappil AK, Zheng S, Zheng B, Yang D, Wang Q. 3,5-DCQA as a Major Molecule in MeJA-Treated Dendropanax morbifera Adventitious Root to Promote Anti-Lung Cancer and Anti-Inflammatory Activities. Biomolecules. 2024 14(6)
105. Venkataesan Kumari B, Mani R, Asokan BR, Balakrishnan K, Ramasamy A, Parthasarathi R. et al. Green Synthesised Silver Nanoparticles Using Anoectochilus elatus Leaf Extract: Characterisation and Evaluation of Antioxidant, Anti-Inflammatory, Antidiabetic, and Antimicrobial Activities. Journal of Composites Science [Internet]. 2023 7(11)
106. Pandey B, Baral R, Kaundinnyayana A, Panta S. Promising hepatoprotective agents from the natural sources: a study of scientific evidence. Egyptian Liver Journal. 2023;13:1-26
107. Zhang H, Jacob JA, Jiang Z, Xu S, Sun K, Zhong Z. et al. Hepatoprotective effect of silver nanoparticles synthesized using aqueous leaf extract of Rhizophora apiculata. Int J Nanomedicine. 2019;14:3517-24
108. Li W, Zhou C, Fu Y, Chen T, Liu X, Zhang Z. et al. Targeted delivery of hyaluronic acid nanomicelles to hepatic stellate cells in hepatic fibrosis rats. Acta Pharmaceutica Sinica B. 2020;10(4):693-710
109. Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD)-pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metabolism Reviews2017.
110. Zhang X, Ji X, Wang Q, Li JZ. New insight into inter-organ crosstalk contributing to the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Protein Cell. 2018;9(2):164-77
111. Bagherniya M, Nobili V, Blesso CN, Sahebkar A. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: A clinical review. Pharmacological Research2018.
112. Feng Q, Liu W, Baker SS, Li H, Chen C, Liu Q. et al. Multi-targeting therapeutic mechanisms of the Chinese herbal medicine QHD in the treatment of non-alcoholic fatty liver disease. Oncotarget. 2017 8(17)
113. Xu Y, Guo W, Zhang C, Chen F, Tan HY, Li S. et al. Herbal Medicine in the Treatment of Non-Alcoholic Fatty Liver Diseases-Efficacy, Action Mechanism, and Clinical Application. Frontiers in Pharmacology2020.
114. Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M. et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nature Reviews Gastroenterology and Hepatology2019.
115. Liu H, Sun Z, Tian X, Feng Q, Guo Z, Chen S. et al. Systematic investigation on the chemical basis of anti-NAFLD Qushi Huayu Fang. Part 1: A study of metabolic profiles in vivo and in vitro by high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Biomedical Chromatography. 2020 34(5)
116. Ren X, Ye D, He W, Wu X, Guo J. Therapeutic roles of a natural herbal product on ameliorating hepatic steatosis via gut dysbacteriosis modulating in treatment of nonalcoholic fatty liver disease in mice. Phytomedicine Plus. 2021 1(4)
117. Drygalski K, Siewko K, Chomentowski A, Odrzygóźdź C, Zalewska A, Krȩtowski A. et al. Phloroglucinol Strengthens the Antioxidant Barrier and Reduces Oxidative/Nitrosative Stress in Nonalcoholic Fatty Liver Disease (NAFLD). Oxidative Medicine and Cellular Longevity. 2021. 2021
118. Xie K, He X, Chen K, Sakao K, Hou DX. Ameliorative effects and molecular mechanisms of vine tea on western diet-induced NAFLD. Food and Function. 2020 11(7)
119. Jazayeri-Tehrani SA, Rezayat SM, Mansouri S, Qorbani M, Alavian SM, Daneshi-Maskooni M. et al. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutrition & Metabolism. 2019;16(1):8
120. Diehl AM, editor Liver disease in alcohol abusers. Clinical perspective. Alcohol. 2002
121. Chacko KR, Reinus J. Spectrum of Alcoholic Liver Disease. Clinics in Liver Disease2016.
122. Ding RB, Tian K, Huang LL, He CW, Jiang Y, Wang YT. et al. Herbal medicines for the prevention of alcoholic liver disease: a review. J Ethnopharmacol. 2012;144(3):457-65
123. Ding Y, Park B, Ye J, Wang X, Liu G, Yang X. et al. Surfactant-Stripped Semiconducting Polymer Micelles for Tumor Theranostics and Deep Tissue Imaging in the NIR-II Window. Small. 2022;18(6):e2104132
124. de Avelar CR, Pereira EM, de Farias Costa PR, de Jesus RP, de Oliveira LPM. Effect of silymarin on biochemical indicators in patients with liver disease: Systematic review with meta-analysis. World J Gastroenterol. 2017;23(27):5004-17
125. Feng R, Chen JH, Liu CH, Xia FB, Xiao Z, Zhang X. et al. A combination of Pueraria lobata and Silybum marianum protects against alcoholic liver disease in mice. Phytomedicine. 2019 58
126. Soleimani D, Paknahad Z, Rouhani MH. Therapeutic effects of garlic on hepatic steatosis in nonalcoholic fatty liver disease patients: A randomized clinical trial. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2020 13
127. Wang Y, Guan M, Zhao X, Li X. Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm Biol. 2018;56(1):325-32
128. Chigurupati H, Auddy B, Biyani M, Stohs SJ. Hepatoprotective Effects of a Proprietary Glycyrrhizin Product during Alcohol Consumption: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Phytotherapy Research. 2016 30(12)
129. Jung JC, Lee YH, Kim SH, Kim KJ, Kim KM, Oh S. et al. Hepatoprotective effect of licorice, the root of Glycyrrhiza uralensis Fischer, in alcohol-induced fatty liver disease. BMC Complementary and Alternative Medicine. 2016 16(1)
130. Bai X, Su G, Zhai S. Recent Advances in Nanomedicine for the Diagnosis and Therapy of Liver Fibrosis. Nanomaterials (Basel). 2020 10(10)
131. Yang AY, Kim K, Kwon HH, Leem J, Song JE. 6-Shogaol Ameliorates Liver Inflammation and Fibrosis in Mice on a Methionine- and Choline-Deficient Diet by Inhibiting Oxidative Stress, Cell Death, and Endoplasmic Reticulum Stress. Molecules. 2024 29(2)
132. Abd El-Emam MM, Mostafa M, Farag AA, Youssef HS, El-Demerdash AS, Bayoumi H. et al. The Potential Effects of Quercetin-Loaded Nanoliposomes on Amoxicillin/Clavulanate-Induced Hepatic Damage: Targeting the SIRT1/Nrf2/NF-κB Signaling Pathway and Microbiota Modulation. Antioxidants [Internet]. 2023 12(8)
133. Bosan A, Qureshi H, Bile KM, Ahmad I, Hafiz R. A review of hepatitis viral infections in Pakistan. Journal of the Pakistan Medical Association2010.
134. Jamali R. Epidemiologic Studies on Viral Hepatitis: A Short Review. Thrita. 2014 3(1)
135. Ogholikhan S, Schwarz KB. Hepatitis vaccines. Vaccines2016.
136. Shi X, Yu L, Zhang Y, Liu Z, Zhang H, Zhang Y. et al. Glycyrrhetinic acid alleviates hepatic inflammation injury in viral hepatitis disease via a HMGB1-TLR4 signaling pathway. International Immunopharmacology. 2020 84
137. Hunter RJ, Preedy VR. (Eds.). Nanomedicine in Health and Disease (1st ed.). CRC Press. 2012
138. Shah NJ, Aloysius MM, Sharma NR, Pallav K. Advances in treatment and prevention of hepatitis B. World J Gastrointest Pharmacol Ther. 2021;12(4):56-78
139. Luo J, Liao J, Wang Y, Zhao J, Xie X, Qu F. et al. Advances in traditional Chinese medicine for liver disease therapy in 2021. Tradit Med Res. 2022;7:50-9
140. Li R, Bao Z, Wang P, Deng Y, Fan J, Zhu X. et al. Gelatin-Functionalized Carbon Nanotubes Loaded with Cisplatin for Anti-Cancer Therapy. Polymers [Internet]. 2023 15(16)
141. Kim SH, Jo SH, Kwon YI, Hwang JK. Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. International Journal of Molecular Sciences. 2011;12(6)
142. Ahangarpour A, Oroojan AA, Khorsandi L, Kouchak M, Badavi M. Solid Lipid Nanoparticles of Myricitrin Have Antioxidant and Antidiabetic Effects on Streptozotocin-Nicotinamide-Induced Diabetic Model and Myotube Cell of Male Mouse. Oxid Med Cell Longev. 2018;2018:7496936
143. Bairagi B, Khan FR, Nath D. Identification of Bacterial Population from Diabetic Wound of Mice to Study the Bactericidal Efficacy of Green Synthesized Silver Nanoparticle by Saraca asoca Bark Extract. BioNanoScience. 2023;13:436-49
144. Zhang Y, Li J, Wang Z, Xu M-Z, Zeng Z, Huang J-p. et al. Natural plant-derived polygalacturonic acid-oleanolic acid assemblies as oral-delivered nanomedicine for insulin resistance treatment. Chemical Engineering Journal. 2020;390:124630
145. Taghipour YD, Hajialyani M, Naseri R, Hesari M, Mohammadi P, Stefanucci A. et al. Nanoformulations of natural products for management of metabolic syndrome. Int J Nanomedicine. 2019;14:5303-21
146. Deng W, Wang H, Wu B, Zhang X. Selenium-layered nanoparticles serving for oral delivery of phytomedicines with hypoglycemic activity to synergistically potentiate the antidiabetic effect. Acta Pharm Sin B. 2019;9(1):74-86
147. Li L, Sheng X, Zhao S, Zou L, Han X, Gong Y. et al. Nanoparticle-encapsulated emodin decreases diabetic neuropathic pain probably via a mechanism involving P2X3 receptor in the dorsal root ganglia. Purinergic Signal. 2017;13(4):559-68
148. Lee YG, Jung JW, Lee H, Seo KH, Lee DY, Kim HG. et al. Flavonoids from Chionanthus retusus (Oleaceae) Flowers and Their Protective Effects against Glutamate-Induced Cell Toxicity in HT22 Cells. International Journal of Molecular Sciences. 2019 20(14)
149. Chen CL, Tsai WH, Chen CJ, Pan TM. Centella asiatica extract protects against amyloid beta(1-40)-induced neurotoxicity in neuronal cells by activating the antioxidative defence system. J Tradit Complement Med. 2016;6(4):362-9
150. Bala M, Gupta V, Prasad J. A standardized Hippophae extract (SBL-1) counters neuronal tissue injuries and changes in neurotransmitters: implications in radiation protection. Pharm Biol. 2017;55(1):1833-42
151. Bavi EP, Shakerinasab E, Hamidinezhad H, Nazifi E. A green and facile approach for fabrication of biocompatible anti-Parkinson chitosan-gelatin-green tea extract composite particles with neuroprotective and Neurotherapeutic effects: In vitro evaluation. Int J Biol Macromol. 2023;224:1183-95
152. Dar NJ, Bhat SA. (Eds.). Small Molecules in Neurodegeneration (1st ed.). 2025
153. Park SY, Yi EH, Kim Y, Park G. Anti-neuroinflammatory effects of Ephedra sinica Stapf extract-capped gold nanoparticles in microglia. Int J Nanomedicine. 2019;14:2861-77
154. Xue Y, Wang N, Zeng Z, Huang J, Xiang Z, Guan Y-Q. Neuroprotective effect of chitosan nanoparticle gene delivery system grafted with acteoside (ACT) in Parkinson's disease models. Journal of Materials Science & Technology. 2020;43:197-207
155. Lohan S, Raza K, Mehta SK, Bhatti GK, Saini S, Singh B. Anti-Alzheimer's potential of berberine using surface decorated multi-walled carbon nanotubes: A preclinical evidence. Int J Pharm. 2017;530(1-2):263-78
156. Vijayakumar S, Vinoj G, Malaikozhundan B, Shanthi S, Vaseeharan B. Plectranthus amboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larvae. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015;137:886-91
157. Murugan K, Benelli G, Panneerselvam C, Subramaniam J, Jeyalalitha T, Dinesh D. et al. Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp Parasitol. 2015;153:129-38
158. Marimuthu S, Rahuman AA, Rajakumar G, Santhoshkumar T, Kirthi AV, Jayaseelan C. et al. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol Res. 2011;108(6):1541-9
159. Aditya NP, Chimote G, Gunalan K, Banerjee R, Patankar S, Madhusudhan B. Curcuminoids-loaded liposomes in combination with arteether protects against Plasmodium berghei infection in mice. Exp Parasitol. 2012;131(3):292-9
160. Ibrahim N, Ibrahim H, Sabater AM, Mazier D, Valentin A, Nepveu F. Artemisinin nanoformulation suitable for intravenous injection: Preparation, characterization and antimalarial activities. Int J Pharm. 2015;495(2):671-9
161. Tang Y, Cao J, Cai Z, An H, Li Y, Peng Y. et al. Epigallocatechin gallate induces chemopreventive effects on rats with diethylnitrosamine-induced liver cancer via inhibition of cell division cycle 25A. Mol Med Rep. 2020;22(5):3873-85
162. Abdalla MO, Karna P, Sajja HK, Mao H, Yates C, Turner T. et al. Enhanced noscapine delivery using uPAR-targeted optical-MR imaging trackable nanoparticles for prostate cancer therapy. J Control Release. 2011;149(3):314-22
163. Cavalcante de Freitas PG, Rodrigues Arruda B, Araújo Mendes MG, Barroso de Freitas JV, da Silva ME, Sampaio TL. et al. Resveratrol-Loaded Polymeric Nanoparticles: The Effects of D-α-Tocopheryl Polyethylene Glycol 1000 Succinate (TPGS) on Physicochemical and Biological Properties against Breast Cancer In vitro and In vivo. Cancers (Basel). 2023 15(10)
164. Alhajri HM, Aloqaili SS, Alterary SS, Alqathama A, Abdalla AN, Alzhrani RM. et al. Olive Leaf Extracts for a Green Synthesis of Silver-Functionalized Multi-Walled Carbon Nanotubes. J Funct Biomater. 2022 13(4)
165. Yang H, Liu R, Xu Y, Qian L, Dai Z. Photosensitizer Nanoparticles Boost Photodynamic Therapy for Pancreatic Cancer Treatment. Nanomicro Lett. 2021;13(1):35
166. Mokbel K, Wazir U, Mokbel K. Chemoprevention of Prostate Cancer by Natural Agents: Evidence from Molecular and Epidemiological Studies. Anticancer Res. 2019;39(10):5231-59
167. Xu B, He Y, Zhang Y, Ma Z, Zhang Y, Song W. In situ Growth of Tunable Gold Nanoparticles by Titania Nanotubes Templated Electrodeposition for Improving Osteogenesis through Modulating Macrophages Polarization. ACS Applied Materials & Interfaces. 2022;14(45):50520-33
168. Palange AL, Di Mascolo D, Carallo C, Gnasso A, Decuzzi P. Lipid-polymer nanoparticles encapsulating curcumin for modulating the vascular deposition of breast cancer cells. Nanomedicine. 2014;10(5):991-1002
169. Elbagory AM, Hussein AA, Meyer M. The In vitro Immunomodulatory Effects Of Gold Nanoparticles Synthesized From Hypoxis hemerocallidea Aqueous Extract And Hypoxoside On Macrophage And Natural Killer Cells. Int J Nanomedicine. 2019;14:9007-18
170. Zhang F, Zhang JG, Qu J, Zhang Q, Prasad C, Wei ZJ. Assessment of anti-cancerous potential of 6-gingerol (Tongling White Ginger) and its synergy with drugs on human cervical adenocarcinoma cells. Food Chem Toxicol. 2017;109(Pt 2):910-22
171. Wu J, Ma X, Lu Y, Zhang T, Du Z, Xu J. et al. Edible Pueraria lobata-Derived Exosomes Promote M2 Macrophage Polarization. Molecules [Internet]. 2022 27(23)
172. De Robertis M, Sarra A, D'Oria V, Mura F, Bordi F, Postorino P, et al. Blueberry-Derived Exosome-Like Nanoparticles Counter the Response to TNF-α-Induced Change on Gene Expression in EA.hy926 Cells. Biomolecules. 2020;10(5)
173. Ahn S, Singh P, Castro-Aceituno V, Yesmin Simu S, Kim YJ, Mathiyalagan R. et al. Gold nanoparticles synthesized using Panax ginseng leaves suppress inflammatory - mediators production via blockade of NF-κB activation in macrophages. Artif Cells Nanomed Biotechnol. 2017;45(2):270-6
174. Wang L, Zhu Z, Han L, Zhao L, Weng J, Yang H. et al. A curcumin derivative, WZ35, suppresses hepatocellular cancer cell growth via downregulating YAP-mediated autophagy. Food & Function. 2019;10(6):3748-57
175. Zhao WJ, Bian YP, Wang QH, Yin F, Yin L, Zhang YL. et al. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol Sin. 2022;43(3):645-58
176. Fan N, Zhao J, Zhao W, Zhang X, Song Q, Shen Y. et al. Celastrol-loaded lactosylated albumin nanoparticles attenuate hepatic steatosis in non-alcoholic fatty liver disease. Journal of Controlled Release. 2022;347:44-54
177. Liang J, Liu Y, Liu J, Li Z, Fan Q, Jiang Z. et al. Chitosan-functionalized lipid-polymer hybrid nanoparticles for oral delivery of silymarin and enhanced lipid-lowering effect in NAFLD. Journal of Nanobiotechnology. 2018;16(1):64
178. Abd-Allah H, Nasr M, Ahmed-Farid OAH, Ibrahim BMM, Bakeer RM, Ahmed RF. Nicotinamide and ascorbic acid nanoparticles against the hepatic insult induced in rats by high fat high fructose diet: A comparative study. Life Sci. 2020;263:118540
179. Mishra D, Jain N, Rajoriya V, Jain AK. Glycyrrhizin conjugated chitosan nanoparticles for hepatocyte-targeted delivery of lamivudine. J Pharm Pharmacol. 2014;66(8):1082-93
180. Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D. et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713
181. Chen JY, Huang TR, Hsu SY, Huang CC, Wang HS, Chang JS. Effect and mechanism of quercetin or quercetin-containing formulas against COVID-19: From bench to bedside. Phytother Res. 2024;38(6):2597-618
182. Stephen Robert JM, Peddha MS, Srivastava AK. Effect of Silymarin and Quercetin in a Miniaturized Scaffold in Wistar Rats against Non-alcoholic Fatty Liver Disease. ACS Omega. 2021;6(32):20735-45
183. Gohari Mahmoudabad A, Gheybi F, Mehrabi M, Masoudi A, Mobasher Z, Vahedi H. et al. Synthesis, characterization and hepatoprotective effect of silymarin phytosome nanoparticles on ethanol-induced hepatotoxicity in rats. Bioimpacts. 2023;13(4):301-11
184. Kędzierska M, Bańkosz M, Drabczyk A, Kudłacik-Kramarczyk S, Jamroży M, Potemski P. Silver Nanoparticles and Glycyrrhiza glabra (Licorice) Root Extract as Modifying Agents of Hydrogels Designed as Innovative Dressings. Int J Mol Sci. 2022 24(1)
185. Bairagi R, Patil S. Glycyrrhiza glabra assisted green synthesis of metallic nanoparticles with different applications: A review. International Journal of Life Science Research Archive. 2023
186. Zhu J, Qin F, Ji Z, Fei W, Tan Z, Hu Y. et al. Mannose-Modified PLGA Nanoparticles for Sustained and Targeted Delivery in Hepatitis B Virus Immunoprophylaxis. AAPS PharmSciTech. 2019;21(1):13
187. Wang W, Zhou X, Bian Y, Wang S, Chai Q, Guo Z. et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat Nanotechnol. 2020;15(5):406-16
188. Li Q, Du YZ, Yuan H, Zhang XG, Miao J, Cui FD. et al. Synthesis of lamivudine stearate and antiviral activity of stearic acid-g-chitosan oligosaccharide polymeric micelles delivery system. Eur J Pharm Sci. 2010;41(3-4):498-507
189. Li LH, Deng JC, Deng HR, Liu ZL, Xin L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr Res. 2010;345(8):994-8
Author contact
![]() Corresponding authors: Avtar Singh Meena, Ph.D. Assistant Professor, Department of Biotechnology, All India Institute of Medical Sciences (AIIMS), Ansari Nagar, New Delhi, India, 110029, Email: avtarjephedu; Shiv Pratap Singh Yadav, Ph.D. Scientist ISB (Biophysics), Division CSIR Centre for Cellular and Molecular Biology (CCMB), Uppal Road, Hyderabad, Telangana, India, 500007; Email: shivpratapres.in.
Corresponding authors: Avtar Singh Meena, Ph.D. Assistant Professor, Department of Biotechnology, All India Institute of Medical Sciences (AIIMS), Ansari Nagar, New Delhi, India, 110029, Email: avtarjephedu; Shiv Pratap Singh Yadav, Ph.D. Scientist ISB (Biophysics), Division CSIR Centre for Cellular and Molecular Biology (CCMB), Uppal Road, Hyderabad, Telangana, India, 500007; Email: shivpratapres.in.

 Global reach, higher impact
Global reach, higher impact