ISSN: 2206-7418
Nanotheranostics 2023; 7(3):327-344. doi:10.7150/ntno.82654 This issue Cite
Review
Engineering molecular nanoprobes to target early atherosclerosis: Precise diagnostic tools and promising therapeutic carriers
1. Department of Nuclear Medicine, First Hospital of Shanxi Medical University, Taiyuan, China
2. Collaborative Innovation Center for Molecular Imaging of Precision Medicine, Shanxi Medical University, Taiyuan, China
Received 2023-1-24; Accepted 2023-3-2; Published 2023-4-2
Abstract
Atherosclerosis, an inflammation-driven chronic blood vessel disease, is a major contributor to devastating cardiovascular events, bringing serious social and economic burdens. Currently, non-invasive diagnostic and therapeutic techniques in combination with novel nanosized materials as well as established molecular targets are under active investigation to develop integrated molecular imaging approaches, precisely visualizing and/or even effectively reversing early-stage plaques. Besides, mechanistic investigation in the past decades provides many potent candidates extensively involved in the initiation and progression of atherosclerosis. Recent hotly-studied imaging nanoprobes for detecting early plaques mainly including optical nanoprobes, photoacoustic nanoprobes, magnetic resonance nanoprobes, positron emission tomography nanoprobes, and other dual- and multi-modality imaging nanoprobes, have been proven to be surface functionalized with important molecular targets, which occupy tailored physical and biological properties for atherogenesis. Of note, these engineering nanoprobes provide long blood-pool residence and specific molecular targeting, which allows efficient recognition of early-stage atherosclerotic plaques and thereby function as a novel type of precise diagnostic tools as well as potential therapeutic carriers of anti-atherosclerosis drugs. There have been no available nanoprobes applied in the clinics so far, although many newly emerged nanoprobes, as exemplified by aggregation-induced emission nanoprobes and TiO2 nanoprobes, have been tested for cell lines in vitro and atherogenic animal models in vivo, achieving good experimental effects. Therefore, there is an urgent call to translate these preclinical results for nanoprobes into clinical trials. For this reason, this review aims to give an overview of currently investigated nanoprobes in the context of atherosclerosis, summarize relevant published studies showing applications of different kinds of formulated nanoprobes in early detection and reverse of plaques, discuss recent advances and some limitations thereof, and provide some insights into the development of the new generation of more precise and efficient molecular nanoprobes, with a critical property of specifically targeting early atherosclerosis.
Keywords: Atherosclerosis, Early-stage, Nanoprobes, Molecular imaging, Single-modality, Dual-modality, Multi-modality, Precise diagnosis, Therapeutics
Introduction
Atherosclerosis, a predominant cause of death and disability worldwide, is well characterized by excessive smouldering inflammation, and lipid metabolism dysfunction in terms of the pathological nature [1-3]. On the one hand, abnormal accumulation of apolipoprotein B-containing lipoproteins, mainly low-density lipoproteins (LDLs) in the arterial intima, contributes to early atherogenesis, and following inflammatory responses exacerbate plaque progression over decades, which can lead to the sudden occurrence of fatal cardiovascular events, including plaque rupture, myocardial infarction, stroke, and even sudden death [4-7]. On the other hand, advanced plaques are very hard to reverse, and no efficient therapies have been recognized by clinicians so far [8-10]. Therefore, monitoring early atherosclerotic progression timely is extremely crucial to reduce these adverse events for patients with cardiovascular diseases.
In fact, there are quite many clinical techniques applied for atherosclerotic plaque monitoring, as exemplified by computed tomography angiography (CTA), contrast-enhanced cardiac computed tomography (CT), intravascular ultrasound (IVUS), etc. [11-13]. In contrast to invasive imaging methods, non-invasive imaging techniques for atherosclerotic plaque detection with undoubted advantages, such as noninvasiveness, precision, high spatiotemporal resolution, and low toxicity, have taken a chief place [14, 15]. So far, the main non-invasive imaging approaches for patients include ultrasound, X-ray, CT, and magnetic resonance imaging (MRI), having different uses [16-18]. Of interest, vascular calcification, a hallmark of atherosclerosis, can be detected by CT, and lipid-rich necrotic core and hemorrhage can be additionally captured by MRI with high sensitivity [19, 20]. However, these conventional imaging approaches are limited to the identification of advanced plaques. In other words, it still lacks an effective technique to specifically recognize early-stage plaques, which can be reversed by drug intervention and/or other preventive measures [21, 22]. In this regard, developing specific and precise detection methods on the basis of current imaging systems for early-stage plaques is an extremely urgent need. To do so, engineering molecular probes have been emerging and becoming popular to visualize plaque-specific molecules involved in early atherogenesis.
Among various molecular probes, nanoprobes, as a novel type of imaging probes and ultrasmall biosensors, have been shown to contribute to the early diagnosis of multiple diseases, such as Alzheimer's disease [23], and most cancers [24-26]. In addition to neurological diseases and cancers, nanoprobes also facilitate the imaging of atheromatous changes through formulating with intracellular and extracellular biomolecules, additionally providing longer blood-pool residence and more specific molecular targeting [27-29]. Based on this, the application of nanoprobes in combination with other imaging techniques, as exemplified by ultrasound and MRI, can together achieve more precise imaging effects of the plaque morphology and even molecular/cellular signatures of the atheroma. On the one hand, formulated nanoprobes, have been utilized in the diagnosis of atherosclerosis by combining nuclides, fluorophores, and receptors for the identification of plaques in the field of cardiac nuclear medicine. On the other hand, nanoparticle-mediated combination therapy of atherosclerosis can be realized by introducing therapeutic lipid-lowering or anti-inflammation drugs into the nanoprobes, as a kind of drug delivery system. Of note, formulated nanoprobes can provide both the location information and the expression levels of disease-associated signature biomolecules in vivo, eventually leading to early diagnosis of atherosclerosis and other cardiovascular diseases, improved treatment strategies, and accurate assessment of treating efficacy.
Based on this background information, this review aims to give a comprehensive overview of currently-studied nanoprobes in the context of atherosclerosis, summarize published experimental studies showing detective and therapeutic effects of different kinds of engineering nanoprobes on early plaques, go through the contribution of some molecular targets to adding specificity of nanoprobes and discuss current progress and some limitations thereof. Through looking into four common types of single-modality nanoprobes, some dual- and multi-model nanoprobes, and potent molecular targets for early-stage plaque detection and reverse, such as inflammatory mediators and lipid metabolism-related factors, some insights have been gained into the design of more precise and efficient nanoprobes for the recognization of plaque-specific molecules, to achieve early diagnosis and prevention of atherosclerosis eventually.
General overview of theranostic applications of engineering nanoprobes in atherosclerosis
With regard to the application of nanoprobes in targeting plaques, we would like to start with the contribution of molecular imaging in this context. As a non-invasive strategy, molecular imaging mainly targets specific molecules on the tissue and cell levels, and especially shows their changes in the pathological states, in order to have an in-depth understanding of the disease mechanisms, develop new pharmacological targets, and provide novel drug candidates [30, 31]. Given the fact that it is an interdisciplinary subject, molecular imaging indeed involves many different subjects including medicine, radiology, biology, materials science, mathematics, and chemistry [32-34]. In terms of atherosclerosis, molecular imaging has the great potential to reveal deeper insights into cardiovascular inflammation and how it evolves over time [35-38]. In addition, the successful application of molecular imaging requires not only advanced imaging equipment, such as CT, MRI, and positron emission tomography (PET), but also the synthesis of safe contrast agents [26, 39, 40]. Of course, efficient imaging probes, as exemplified by formulated nanoprobes, are extremely important as well. Therefore, considering the potential of nanoprobes in the early detection and diagnosis of atherosclerosis as well as the necessity of targeting early plaques, it is preferable for researchers to develop more sensitive and specific nanoprobes, by which targeted contrast agents can be formed together for thrombosis and plaque imaging.
Proposed applications of engineering nanoprobes in atherosclerosis are mainly divided into two types. First of all, various preclinical studies have shown that formulated nanoprobes can specifically recognize early-stage plaques through targeting plaque-specific molecules. Detection of nanoprobes can be accomplished by a variety of methods. Moreover, the successful imaging of monocytes, macrophages, foam cells, and other plaque components, has enabled these nanoprobes to become promising in realizing the visualization of plaques, especially in the early stage of atherosclerosis [41]. Secondly, nanoprobes can act as efficient carriers for anti-inflammation and anti-lipid metabolism drug delivery, assisting in achieving therapeutic purposes [41, 42]. In this sense, organic nanoparticles are classical examples to provide both platforms for improved detection (molecular imaging) and more efficacious treatment (drug delivery) of atherosclerosis, owing to their intrinsic physical properties, i.e. superior biocompatibility, and drug-loading capacity [41, 43, 44]. To sum up, these engineering nanoprobes can function as potential carriers for both imaging and therapeutic agents for atherosclerotic plaques, in turn extending their traditional clinical applications in this field.
More interestingly, new dual-mode imaging formed by the fusion of multiple ones, such as optical/MR dual-model imaging, optical/ultrasound dual-modality imaging, and photoacoustic/ultrasound imaging and others, has attracted wide attention and tested for atherosclerotic animal models [27, 45-47], as single-mode imaging is not enough to collect accurate imaging information. In addition to overcoming the limitations of single-mode imaging, multi-mode imaging can achieve better imaging effects through combining the advantages of various imaging modalities, to realize the early diagnosis of atherosclerosis. Of note, those fabricated multimodal imaging nanoparticles with reactive oxygen species (ROS)-scavenging ability have been reported to provide a new avenue for the diagnosis and treatment of vulnerable plaques, constructing a novel theranostic nanoplatform for atherosclerosis [48]. Overall, recent advances in imaging and treatment of atherosclerosis based on various nanoprobes bring a couple of new opportunities in the future. In the next section, theranostic applications of different kinds of engineering nanoprobes in atherosclerosis will be described in detail according to the number of modalities, with the special focus on comparison of different targeting strategy.
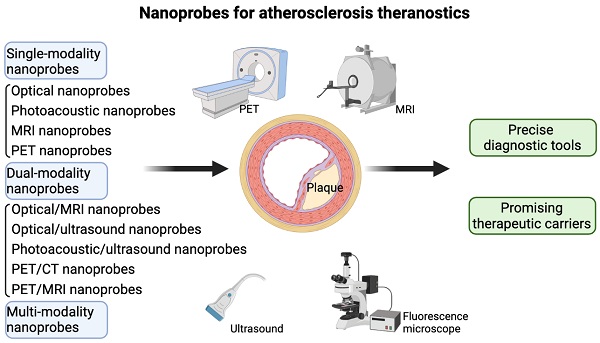
Single-modality imaging nanoprobes for early detection and prevention of plaques
To the best of our knowledge, there has been no systematic review reported for recent advances in formulating nanoprobes in the context of atherosclerosis so far. In this review, comparisons of different kinds of nanoprobes for atherosclerotic plaques have been listed in Table 1, covering their major advantages as well as disadvantages. Currently investigated single-modality molecular nanoprobes for plaque detection mainly include optical imaging nanoprobes, photoacoustic imaging nanoprobes, magnetic resonance imaging nanoprobes, and positron emission tomography imaging nanoprobes, which have been summarized in Table 2 and Figure 1, and will be discussed in the following subchapters. Moreover, newly emerging dual-modality imaging nanoprobes will be described in the next section, and summed up in Table 3 and Figure 2. Taken together, these theranostic applications of these single- and/or dual-modality imaging nanoprobes in atherosclerosis, demonstrated by many recent original studies, would give more hope for early diagnosis and prevention of patients with plaques in clinical practice.
Comparisons of four major types of single-modality nanoprobes for plaque detection
| Types | Detection | Examples | Main advantage | Main disadvantage |
|---|---|---|---|---|
| Optical imaging nanoprobes | Fluorescence | AIE nanoprobes; luminescence nanoprobes | High contrast agent sensitivity | Low tissue penetration |
| Photoacoustic imaging nanoprobes | Ultrasonic waves | TiO2 nanoprobes | High contrast and deep tissue penetration | Toxicity |
| MR imaging nanoprobes | Magnetic field radio waves | Tissue factor-targeting magnetic nanoprobes | High spatial resolution | Difficult to quantify |
| PET imaging nanoprobes | γ-ray | DOTA-CANF-comb nanoprobes | High sensitivity | Radionuclides must be used |
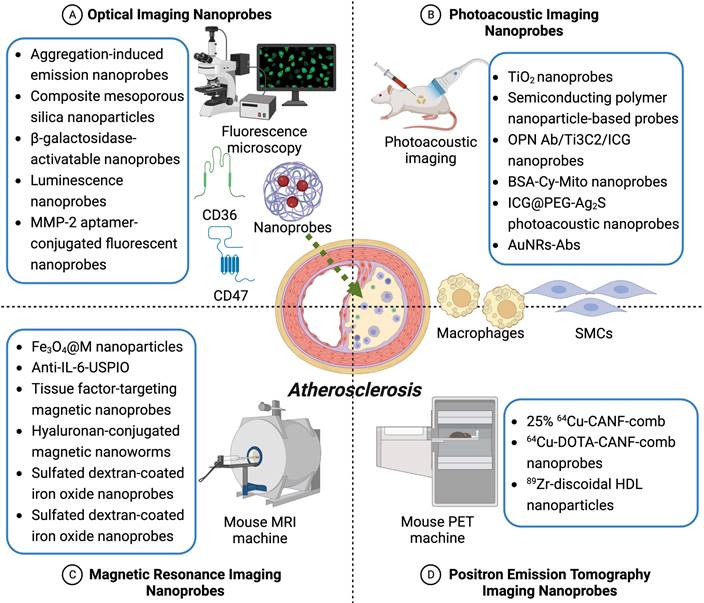
Optical imaging nanoprobes in atherosclerotic research
In the scope of optical imaging, fluorescent materials for labeling, including nano fluorescent probes, have been widely applied to develop optical imaging technology, providing new approaches for early monitoring and treatment of some diseases, such as cancers [49-51]. Given the high spatiotemporal resolution as well as the high sensitivity of optical techniques in comparison with other imaging platforms, the application of optical nanoparticles in cardiovascular research has been gradually increasing, as comprehensively reviewed by several recent articles [52-54]. As for atherosclerotic studies, optical imaging nanoprobes have multiple advantages for plaque detection, for example, high contrast agent sensitivity, and probe versatility. In addition, optical imaging nanoprobes are very fast and efficient. However, the anatomical information is hard to collect and the quantification is difficult to make, which are the main limitations of optical imaging nanoprobes [26]. Even so, there are some original studies to investigate the effects of engineering nanoprobes on detecting and preventing atherosclerotic plaques, highlighting their potential in molecular imaging of atherosclerosis.
Wang and colleagues have recently developed a kind of highly bright aggregation-induced emission (AIE) nanoprobes, which are designed to functionalize with anti-cluster of differentiation (CD) 47 antibodies, to detect early-stage plaques in Apolipoprotein E-deficient (Apoe-/-) mice [28]. CD47, as an anti-phagocytic signal for macrophages, has been confirmed to contribute to atherogenesis [55, 56]. Of note, CD47-blocking antibodies have been found to restore phagocytosis and meanwhile protect against atherosclerosis in multiple animal models, mechanistically through the regulation of pro-atherosclerotic factor, and tumor necrosis factor (TNF)-α [57, 58]. Based on this, these nanoprobes formulated with anti-CD47 antibodies can specifically bind to CD47 overexpressed in atherosclerotic plaques, allowing the efficient recognition of plaques at different stages, especially for the identification of early-stage plaques prior to CT and MRI. Moreover, the clinical use of this kind of fluorescent nanoprobes in targeted imaging of human carotid plaques has been also demonstrated in their study [28]. These findings together suggest the potential value of these AIE nanoprobes in monitoring the therapeutic effects of anti-atherosclerosis drugs. In addition to CD47, CD36 is the other target used to develop novel nanoprobes in the context of atherogenesis. Oxidized LDL/CD36 signaling in macrophages has been shown to drive chronic inflammation by mediating dysregulated fatty acid metabolism and oxidative stress from the mitochondria [59, 60]. Sun et al. have applied CD36-antibody-modified-luminescence nanoprobes for in situ imaging of CD36 activation as well as CD36-ox-LDL binding in plaque-associated macrophages with high sensitivity and good stability, which has not been directly visualized in living macrophages by other imaging tools [61]. Of interest, the ROS signaling has been observed to enhance the binding of ox-LDL to CD36 in this process, demonstrating new atherogenesis signaling at the cellular level from a different angle [61].
Given the fact that matrix metalloproteinase-2 (MMP-2) contributes to atherogenesis through affecting the functional activities of immune cells, endothelial cells, vascular smooth muscle cells (VSMCs), and platelets [62-64], Han et al. have tested an MMP-2-specific aptamer-conjugated fluorescent nanoprobe in Apoe-/- mice to visualize atherosclerotic plaques [65]. They have successfully constructed this kind of fluorescent nanoprobe using a modified DNA SELEX technique and simultaneously achieved good effects through ex vivo imaging. It is worth mentioning that this nanoprobe may be available not only for atherosclerotic plaque imaging, but also for gastric cancer tissue visualization. Because MMP-2 aptamer could detect MMP-2 expression in both types of tissues [65]. Moreover, considering that atherosclerosis also belongs to aging-associated diseases, β‐galactosidase-activatable nanoprobes, showing good accumulation in arteries, have been developed for in vivo imaging of senescent vascular cells in atherosclerotic mice [66]. More interestingly, this is the first original study to obtain the successful in vivo imaging of senescent cells in pathological vasculatures, although there are several fluorescent probes reported due to the overexpression of senescence-associated β-galactosidases in senescent cells [67, 68]. In addition to the accurate identification of plaques, the designed CMSN@SRT@Anti nanoprobes have also shown great potential for targeted therapy of atherosclerotic diseases, due to their excellent biocompatibility, high performance, and superior plaque-targeting ability. After a four-week post-treatment of CMSN@SRT@Anti, persistent fluorescence signals were observed in atherosclerotic lesions, and the aortic plaque area was significantly reduced in Apoe-/- mice [69]. Collectively, the above-mentioned five studies involve different molecular targets of atherosclerosis, and these nanoprobes are conjugated with the same single imaging technique, i.e. optical imaging.
In summary, optical imaging nanoprobes are one type of the most commonly-studied nanoprobes for atherosclerotic plaque detection. So far, CD47, CD36, β‐galactosidase, MMP-2, and osteopontin have been targeted and further validated both in vivo and in vitro. Even though these formulated nanoprobes are still limited to preclinical exploration, current preliminary data provide a solid basis for their clinical application. In addition to these molecular targets discussed in the above paragraphs, other key players in atherogenesis, such as inflammatory mediators, classical and atypical chemokines, receptors, and lipid metabolism-related factors should be also included in future studies, which would offer more choices for designing more sensitive and feasible nanoprobes under the guidance of advanced imaging systems.
Photoacoustic imaging nanoprobes in atherosclerotic research
As a non-invasive and non-ionizing biomedical imaging method, photoacoustic imaging (PAI) technology, combining the acoustic resolution and imaging depth of ultrasonography with the sensitivity of optical imaging, has been well developed and applied in the field of atherosclerosis research in the past decade [53, 70, 71]. To achieve more specific and high-sensitivity imaging effects of lesional area, PAI nanoprobes have been introduced for plaque detection, with strong light absorption properties, mainly encompassing metal sulfur/selenium/carbide, carbon-based nanoprobes, gold-based nanoprobes, black phosphorus, organic small molecules, for example, indocyanine green (ICG) and melanin, as systematically summarized by two recent review articles [26, 72]. Importantly, PAI nanoprobes are beneficial for the differentiation of some specific contents of disease tissues from the control tissues at the molecular level. Of interest, intravascular photoacoustic imaging (IVPA), as a new tool, has shown great potential to visualize both plaque structure and composition, especially for lipid-rich vulnerable plaques [73-75]. In this section, we will mainly discuss some relevant original studies investigating newly-designed PAI nanoprobes for atherosclerotic plaque detection and even their therapeutic potential, which have been detailed in Table 2.
Single-modality imaging nanoprobes for early detection and prevention of atherosclerotic plaques. Depicted are four common types of single-modality imaging nanoprobes applied in atherosclerosis research, including optical imaging nanoprobes, photoacoustic imaging nanoprobes, magnetic resonance imaging nanoprobes, and positron emission tomography imaging nanoprobes. Representative nanoprobes in each category are listed in detail here according to current studies.
Studies investigating applications of single-modality nanoprobes in atherosclerosis research
| Imaging properties | Nanoprobes | Models | Targets | Mechanisms | Applications | References |
|---|---|---|---|---|---|---|
| Optical imaging nanoprobes | Aggregation-induced emission nanoprobes | Apoe-/- mice | CD47 | Regulation of the substituent of rhodanine | Early detection of plaques and screening of anti-AS drugs | [28] |
| Composite mesoporous silica nanoparticles | Apoe-/- mice; RAW264.7 cells | CD36 | Targeting macrophages | Inhibition and imaging of plaques | [69] | |
| β-galactosidase-activatable nanoprobes | Apoe-/- mice | β-galactosidase | Targeting senescent vascular cells | Early diagnosis and therapy of AS | [66] | |
| Luminescence nanoprobes | Apoe-/- mice | CD36 activation, CD36-oxLDL binding | Targeting foam cell formation | Monitoring the progression of atherogenesis | [61] | |
| MMP-2 aptamer-conjugated fluorescent nanoprobes | Apoe-/- mice | MMP-2 | Targeting MMP-2 protein | Diagnostic tools of AS and cancer | [65] | |
| Photoacoustic imaging nanoprobes | TiO2 nanoprobes | RAW 264.7 cells | Intracellular lipids | Cholesterol regulation pathways | Mild phototherapy | [29] |
| Semiconducting polymer nanoparticle-based probes | Apoe-/- mice | CD36 | Targeting inflammation of carotid plaques | Non-invasive imaging and assessment of plaques | [80] | |
| OPN Ab/Ti3C2/ICG nanoprobes | Apoe-/- mice | Osteopontin | Targeting VASPs | Differentiation of VASPs | [79] | |
| BSA-Cy-Mito nanoprobes | Apoe-/- mice | BSA | Targeting ox-LDL-activated macrophages | Early identification of rupture-prone plaques | [78] | |
| ICG@PEG-Ag2S photoacoustic nanoprobes | Apoe-/- mice | C18/PEG polymer molecules | Due to the lipophilicity of the C18 chain to AS microenvironment | Imaging of plaques | [76] | |
| AuNRs-Abs | Atherosclerotic rabbits; HUVECs | MMP-2 | Targeting inflammation | Imaging of plaques | [77] | |
| MR imaging nanoprobes | Fe3O4@M nanoparticles | Wistar rats | VCAM-1 | The specific recognition of integrin α4β1 to VCAM-1 | Diagnosis of early stage plaques | [88] |
| Anti-IL-6-USPIO | Atherosclerotic rabbits; HUVECs | IL-6 | Targeting inflammatory cytokines | Imaging of VASPs | [87] | |
| Tissue factor-targeting magnetic nanoprobes | Apoe-/- mice | Tissue factor | Targeting TF-positive atherosclerotic plaques | Detection of plaques | [86] | |
| Hyaluronan-conjugated magnetic nanoworms | Apoe-/- mice | CD44 | Targeting CD44-expressing cells in plaques | Detection of plaques | [92] | |
| Sulfated dextran-coated iron oxide nanoprobes | J774 macrophages | SR-A | Targeting macrophages | Imaging of VASPs | [95] | |
| Gadolinium immunonanoparticle-based nanoprobes | Apoe-/- mice | MRP 8/14 complex | Targeting inflammation | Potential therapy of atherosclerosis | [91] | |
| PET imaging nanoprobes | 25% 64Cu-CANF-comb | C57BL/6 mice; Apoe-/- mice | NPR-C | Targeting NPR-C expression | Detection of plaques | [103] |
| 64Cu-DOTA-CANF-comb nanoprobes | Murine HLI Model | NPR-C receptors | Targeting angiogenesis | Imaging NPR-C receptor in angiogenesis | [100, 101] | |
| 89Zr-discoidal HDL nanoparticles | Apoe-/- mice; rabbits; pigs | HDL | Targeting plaque macrophages and monocytes | Imaging of plaques | [102] |
Wu and coworkers have reported a type of ICG@PEG-Ag2S PAI nanoprobes for plaque imaging in Apoe-/- mice, showing relatively long blood retention as well as selective accumulation in plaques due to the lipophilicity of the C18 chain to the atherosclerotic microenvironment [76]. Of interest, ICG@PEG-Ag2S PAI nanoprobes have good hemocompatibility and no side effects on the main organs, as shown by hemolysis and coagulation assays. These results together support this fabricated nanoprobe as an available noninvasive imaging tool for atherosclerotic plaques in vivo [76]. In the same year, Qin and colleagues applied MMP-2-targeted gold nanorods for IVPA of atherosclerotic plaques, encouraging their further development for early diagnosis of atherosclerosis [77]. Moreover, two recent experimental studies from Gao et al. and Ge et al. have developed two kinds of nanoprobes to visualize vulnerable atherosclerotic plaques (VASPs) [78, 79]. Gao et al. used the self-assembly of bovine serum albumin (BSA), to construct a kind of BSA-Cy-Mito nanoprobe as a GSH/H2O2 indicator for in vivo photoacoustic imaging of redox status in ox-LDL-activated macrophages as well as high fat diet-fed Apoe-/- mice, in order to evaluate VASP formation with high accuracy. According to their redox states among different types of plaques, BSA-Cy-Mito nanoprobes could specifically distinguish vulnerable and stable plaques, indicating this sensitive redox-responsive PAI nanoprobe may act as a powerful tool for early identification of rupture-prone plaques [78]. In a similar vein, Ge et al. also focused on features of VASPs using another type of nanoprobes, i.e. formulated osteopontin (OPN) Ab/Ti3C2/ICG nanoprobes. In addition to the differentiation of VASPs, OPN Ab/Ti3C2/ICG nanoprobes also highlight the importance of OPN in the non-invasively specific imaging of VASPs at the molecular level [79].
Besides, Xie and colleagues have applied a kind of semiconducting polymer nanoparticle-based probe, to specifically identify inflammatory components involved in atherogenesis and further evaluate the inflammation severity by utilizing atherogenic mouse models. Consistent with experimental findings obtained via luminescence nanoprobes formulated by Sun et al. and described in the above subchapter, Xie et al. also target CD36 and identify the CD36 positive expression as the inflammation level [80]. They have demonstrated that the quantification of the PAI signals can reflect the expression of CD36, and the inflammation severity, with good accuracy. At last, TiO2-HA-p nanoprobes have been reported as a classical example of metal-based nanoprobes applied in experimental studies for atherosclerosis on the basis of PAI [29]. This formulated nanoprobe can specifically target macrophage-derived foam cells, with good photothermal and photodynamic properties as well as excellent biocompatibility. Of special note, black TiO2-HA-p nanoprobe-elicited mild phototherapy leads to decreased intracellular lipid levels in foam cells, mechanistically through the regulation of the SREBP2/LDLR pathway as well as ABCA1-mediated cholesterol efflux. This study clearly addresses the therapeutic potential of black TiO2-HA-p nanoprobes in atherosclerosis [29].
As demonstrated by the above several studies, PAI nanoprobes have been popularly investigated in the context of atherogenesis. With the advantages of optical imaging and ultrasonography, nanoprobe-based PA imaging is a preferable solution to screen the critical ingredients of atherosclerotic plaques at the molecule level, providing many opportunities for further exploring novel noninvasive imaging techniques of deeper tissues, such as human deeper coronary arteries. Targeting MMP-2, osteopontin, CD36, etc., would further improve the specificity of these PAI nanoprobes, which helps to differentiate advanced vulnerable plaques from early-stage lesions on the basis of the image pattern and the degree of contrast enhancement. However, we have to acknowledge that optical/photoacoustic imaging nanoprobes have been only applied in cell lines and experimental animals due to some limitations of the living biological imaging system. They are often enriched in the liver and difficult to metabolize, which leads to strong background signals and poor imaging quality, preventing them from entering the clinic.
Magnetic resonance imaging nanoprobes in atherosclerotic research
To date, atherosclerotic plaques and their major components, as exemplified by pro-inflammatory macrophages, have also been extensively characterized by magnetic resonance imaging (MRI), which can generate images with high spatial resolution and excellent soft-tissue contrast [53, 81, 82]. Of special interest, superparamagnetic iron oxide nanoparticles (SPIONs), as a type of commonly used MRI contrast agent, show excellent biocompatibility [83-85]. However, their specificity is limited and needs to be improved. To tickle this problem, nanoparticle-based imaging contrast agents, in combination with surface-coated molecular targets, such as tissue factor (TF), interleukin (IL)-6, and others, can specifically target the SPIONs to the corresponding epitopes on the macrophage surface, increasing their accumulation in vulnerable plaques, and further improving the accurate detection of atherosclerotic plaques [86-88].
Given the fact that TF is a key proatherogenic factor [89], Wei et al. designed EGFP-EGF1-SPIONs as TF-targeted magnetic nanoprobes to precisely and specifically detect TF-expressing cells, such as monocytes/macrophages, endothelial cells, and/or smooth muscle cells in atherosclerotic plaques, which may support to monitor the incidence of early cardiovascular and cerebrovascular events driven by rupturing plaques [86]. Of note, the transverse relaxation time (T2) of EGFP-EGF1-SPIONs was remarkably reduced compared with that of SPIONs, indicating that EGFP-EGF1-SPIONs are more suitable negative MRI contrast agents than SPIONs in T2-weighted imaging. Consistent with immunohistochemical quantitative analysis, the TF signal intensity showed a dramatic reduction when plaques progress [86]. Huang et al. have formulated Fe3O4@M nanoprobes by coating Fe3O4 biomimetic nanoparticles with the macrophage membrane, which can effectively target early atherosclerotic lesions by the specific recognition of integrin α4β1 to vascular cell adhesion molecule-1 (VCAM-1) [88]. Of interest, the coat of the macrophage membrane on the one hand improves the dispersibility and biosafety of Fe3O4@M nanoprobes, on the other hand, supports specifically recognizing and binding to VCAM-1 through their highly expressed α4β1 integrin. These features together ensure Fe3O4@M nanoprobes as a suitable imaging tool for early plaque detection [88].
Moreover, Maiseyeu et al. have developed a kind of gadolinium immunonanoparticle-based nanoprobe via targeting inflammation-associated myeloid-related protein (MRP) 8/14, which is an extracellularly secreted protein involved in atherogenesis [90, 91]. Chow diet-fed C57BL/6 mice did not show any aortic wall enhancement after anti-MRP-nanoprobe injection, confirming the specificity of these nanoprobes to inflammatory atherosclerotic vessels. Interestingly, MRP-activated macrophages were found to secrete proinflammatory cytokines, and this effect could be reversed by the pretreatment with anti-MRP-nanoprobes, indicating their theranostic potential [91]. More recently, Hossaini Nasr and colleagues reported a novel type of hyaluronan-conjugated iron oxide nanoworm (HA-NW) to target CD44-expressing cells [92]. CD44 has been found to be overexpressed in plaques, and the CD44-HA axis plays a very important role in atherogenic progression [93, 94]. In the meanwhile, HA-NWs display much stronger interactions with CD44-expressing cells in CD44- and HA-dependent manners in comparison with the traditional spherical HA-bearing nanoparticles. Furthermore, successful MRI imaging of plaques by applying HA-NWs has been observed in Apoe-/- mice in vivo [92]. Tang and coworkers have developed sulfated dextran-coated iron oxide nanoparticles with specific targeting to macrophages, and 111In3+ radiolabeled probes were found to bind to the macrophage scavenger receptor A (SR-A) with high-affinity through in vitro characterizations. Additionally, higher levels of surface sulfation lead to much higher uptake efficiency by macrophages, as shown by cell uptake studies, revealing a new standard metric for targeted nanomaterials [95]. Mo and colleagues have synthesized IL-6-targeted superparamagnetic iron oxide nanoparticles (Anti-IL-6-USPIO), and shown optimized MRI detection of vulnerable plaques in atherosclerotic rabbits [87]. Taken together, these studies based on different animal models including mice and rabbits highlight extensive applications of MRI nanoprobes for plaque detection, thereby pointing out a high likelihood of their clinical translation.
To sum up, MRI nanoprobes have shown good prospects for the early diagnosis and treatment of experimental atherosclerosis, even though there are several disadvantages, for example, small molecular weight, short half-life, and high toxicity of some molecular probes. Even so, in comparison to optical/photoacoustic nanoprobes, MRI nanoprobes have a higher possibility to achieve clinical translation, without limitations of equipment. We believe recently-investigated MRI nanoprobes conjugated with different molecular targets would provide more choices for specific recognization and comprehensive evaluation of early-stage plaques.
Positron emission tomography imaging nanoprobes in atherosclerotic research
Positron emission tomography (PET) is particularly suitable for the non-invasive and quantitative characterization of macrophage-mediated inflammation, vascular calcifications as well as angiogenesis in atherosclerosis owing to the high tissue penetration and superior sensitivity [96-98]. However, current PET imaging agents are not targeted. For example, (18)F-fluorodeoxyglucose (18F-FDG) lacks specificity for certain cell types, which can be improved by using nanoparticle-based PET imaging agents that has good targeting properties for atherosclerotic plaques.
So far, there are several studies to apply PET imaging nanoprobes in atherosclerotic research, including single-modality as well as dual-modality nanoprobes. In this section, single-model PET imaging nanoprobes are mainly introduced here. Given that angiogenesis is a complex biologic process in atherosclerosis [99], Liu and colleagues constructed a C-type atrial natriuretic factor (CANF)-conjugated nanoprobe, i.e. DOTA-CANF-comb nanoprobe, to detect natriuretic peptide clearance receptor (NPR-C) levels with PET in an animal model with atherosclerosis-like lesions [100]. The targeted DOTA-CANF-comb nanoprobe shows significantly higher tracer accumulation in comparison with either the nontargeted control nanoprobe or the CANF peptide tracer, as demonstrated by PET imaging. The following immunohistochemistry confirms the upregulation of NPR-C in the angiogenic lesion, which is colocalized in both endothelial and smooth muscle cells [100]. Afterwards, the same research group validated the application of this nanoprobe in atherosclerosis imaging ex vivo and in vivo, and further addressed its potential for clinical translation [101].
Considering that high-density lipoprotein (HDL) is a natural nanoparticle that interacts with macrophages in atherosclerotic plaques, Pérez-Medina et al. developed 89Zr-HDL nanoparticles in combination with noninvasive PET imaging, to visualize its accumulation in advanced plaques. Of special interest, this formulating nanoprobe has been validated in different kinds of atherosclerotic animal models, such as Apoe-/- mice, rabbits, and pigs [102]. Woodard and colleagues applied three types of 64Cu-CANF-comb nanoprobes to assess the in vivo PET imaging of NPR-C, which is expressed on atherosclerotic plaques, and found that the 25% 64Cu-CANF-comb shows the best NPR-C targeting specificity as well as sensitivity in Apoe-/- mice, suggesting the 25% 64Cu-CANF-comb as a good PET imaging agent to detect atherosclerosis [103]. Therefore, these three studies show various designs of PET imaging nanoprobes in terms of different features of atherosclerosis, i.e. angiogenesis, lipoproteins, and inflammatory macrophages.
Dual-modality imaging nanoprobes for early detection and prevention of plaques
To date, dual-modality imaging nanoprobes have been becoming popular, as single-mode imaging is not enough to collect accurate imaging information of plaques. Of note, dual- or multi-modality simultaneous imaging techniques facilitate the integration of information on both anatomy and function, and thus have the potential to improve diagnostic and prognostic evaluation for atherosclerosis [104-106]. The most common pattern of dual-modality imaging nanoprobes for plaque visualization is optical/MR dual-model imaging nanoprobes, and other patterns such as optical/ultrasound, photoacoustic/ultrasound, PET/MR, and PET/CT dual-modality imaging nanoprobes, as summarized in Table 3 and Figure 2 (partially), have also been discussed in this section.
Studies investigating applications of dual-modality nanoprobes in atherosclerosis research
| Imaging properties | Nanoprobes | Models | Targets | Mechanisms | Applications | References |
|---|---|---|---|---|---|---|
| Optical/MR imaging nanoprobes | TPZ/IR780@ HSAeOPN nanoprobes | Apoe-/- mice | Osteopontin | Targeting VASPs | Identification of VASPs and regression of plaques | [27] |
| VEGFR2-targeted upconversion nanoprobes | Apoe-/- mice; HUVECs | VEGFR2 | Targeting angiogenesis | Imaging of VASPs | [119] | |
| ROS-Scavenging Nanoparticles | Apoe-/- mice; RAW264.7 cells | ROS scavengers | Targeting macrophages | Imaging and anti-ROS treatment of VASPs | [48] | |
| MARCO-targeted upconversion luminescence probes | Apoe-/- mice; BMMCs | MARCO | Targeting M1 macrophage polarization | Imaging of VASPs | [46] | |
| PP1-Au@GSH@Gd NCs | Apoe-/- mice; RAW264.7 cells | SR-AI | Targeting foam macrophages | Imaging of VASPs | [118] | |
| Fe3O4 nanoparticle-based probes | Apoe-/- mice | Osteopontin | Targeting foamy macrophages | Imaging of VASPs | [111] | |
| OPN-specific upconversion luminescent probes | Apoe-/- mice; Raw 264.7 cells | Osteopontin | Targeting foamy macrophages | Imaging of VASPs | [114] | |
| PC-NPs | Apoe-/- mice; MOVAS | Profilin-1 | Targeting VSMCs | Detection of plaques | [120] | |
| Fluorescent iron oxide magnetic nanoprobes | Apoe-/- mice; RAW264.7 cells | Folate receptor | Targeting activated macrophages | Detection of FRβ-enriched inflammatory plaques | [45] | |
| Optical/ ultrasound imaging nanoprobes | COP-NP-based nanoprobes | Apoe-/- mice | Osteopontin | Targeting VASPs | Imaging of VSMCs and foam cells | [123] |
| Photoacoustic/ ultrasound imaging nanoprobes | RGDfk peptide-targeted BSA-based nanoprobes | Rabbits | RGDfk peptide | Targeting new blood vessels in vulnerable plaques | Visualization of VASPs | [47] |
| Silica-coated gold nanorods | J774A.1; patients | - | Targeting activated macrophages | Detection and temperature monitoring plaques | [126] | |
| PET/CT imaging nanoprobes | 64Cu-DAPTA-comb | Apoe-/- mice | CCR5 | Characterizing CCR5 expression | Imaging the progression and regression of plaques | [132] |
| 64Cu-vMIP-II-comb | Apoe-/- mice | Chemokine receptors | Detection of chemokine receptors | Assessment of plaque progression | [131] | |
| 64Cu-DOTA-DAPTA-comb | C57BL/6 mice; Apoe-/- mice | CCR5 | Targeting CCR5 | Imaging CCR5 expression in plaques | [130] | |
| PET/MR imaging nanoprobes | 64Cu-MMP2cNPs | CL57/BL6 mice | MMP-2 | Targeting macrophages | Detection of MMP-2 in plaques | [137] |
| 68Ga-HAP-multitag nanoprobes | Apoe-/- mice | HAP | Characterizing vascular calcifications in plaques | Imaging of VASPs | [97] | |
| 68Ga-iron oxide nano-radiomaterials | Apoe-/- mice | OxLDL | Targeting oxidized phospholipids | Detection of plaques | [139] | |
| 68Ga-NGD-MNPs | Rabbits | GEBP11 Peptide | Targeting angiogenesis | Imaging of VASPs | [138] | |
| 64Cu-dextran sulfate coated iron oxide nanoparticles | SD rats; Apoe-/- mice | Macrophages | Targeting vascular inflammation | Identification of vulnerable plaques | [136] |
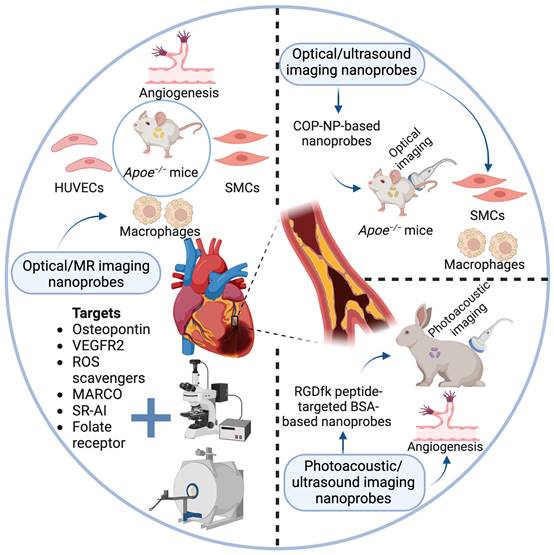
Optical and photoacoustic-based dual-modality imaging nanoprobes for early detection and prevention of plaques. Described are three reported types of optical and photoacoustic-based dual-modality imaging nanoprobes applied in current atherosclerotic studies, mainly including optical/MR imaging nanoprobes, optical/ultrasound imaging nanoprobes, and photoacoustic/ ultrasound imaging nanoprobes. Specific molecular targets and biological processes are displayed.
Optical/MR dual-model imaging nanoprobes in atherosclerotic research
In addition to the application of single optical imaging, the hybrid optical/MR imaging system is also involved in atherosclerotic research. MRI is applied to collect the anatomical information of the diseased blood vessels, and optical fluorescence imaging can further obtain cellular and molecular information due to its high sensitivity [107-110]. Therefore, this bimodal imaging can offer a more precise mode for vulnerable plaques, which has been reported to coordinate with TPZ/IR780@HSAeOPN nanoprobes or Fe3O4 nanoparticle-based probes, and introduced in this subchapter as well [27, 111]. Both nanoprobes target osteopontin, which shows a high expression pattern in plaques, and is closely associated with smooth muscle cell proliferation and foamy macrophage formation [112, 113]. Based on this rationale, Xu together with colleagues established osteopontin-targeted theranostic nanoprobes, which can precisely regress VASPs through a cascade of synergistic events triggered by local irradiation of lasers under the guidance of fluorescence/MR imaging. This hybrid imaging system on the one hand supports that the osteopontin-targeted TPZ/IR780@HSAeOPN nanoprobes could selectively accumulate in the VASP lesions, on the other hand, enables the precise near-infrared (NIR) laser irradiation to generate massive ROS, which results in efficient plaque ablation and amplified hypoxia within VASPs [27]. In a similar vein, Qiao et al. have applied osteopontin-targeted probes based on Fe3O4 nanoparticles to achieve MRI/optical dual-modality imaging of VASPs [111]. It is worth noting that Fe3O4 nanoparticle-based probes have low toxicity, which makes them available in the noninvasive evaluation of early plaques. In their study, these formulated nanoprobes have been found to specifically recognize foamy macrophages through in vitro cell experiments, and further visualize vulnerable plaques as demonstrated by in vivo Apoe-/- mouse model studies [111]. The same research team tested the validity of another OPN-specific upconversion luminescent probe (UCNP-anti-OPN) based on the same mechanism [114].
Owing to that macrophages are key components of atherosclerotic plaques, several researchers have developed dual-modality nanoprobes using molecular targets related to macrophage activation, which would be discussed in detail in this section. Yao and colleagues targeted folate receptor (FR)-β, and formulated a dual-modal fluorescent/MRI contrast agent to detect inflammation-associated activated macrophages within carotid plaques [45]. The reason why they chose FR-β in this study is that FR-β is a specific marker of macrophage activation, and FR-expressing macrophages kind of represent plaque area [115-117]. Additionally, M1 macrophage polarization in plaques has been visualized by optical/MRI dual-modality imaging with MARCO-targeted upconversion luminescence probes, displaying the behavior of M1 phenotype macrophages in Apoe-/- mice [46]. Another studied target associated with macrophage activation is scavenger receptor-AI (SR-AI), which has been conjugated with ultrasmall gold nanoclusters to facilitate dual-modality imaging of vulnerable plaques [118].
In addition to macrophage activation, Fang and coworkers mainly looked into biomarkers associated with the angiogenesis process, and eventually achieved dual-modality imaging of unstable plaques via utilizing VEGFR2-targeted upconversion nanoprobes. Of note, FITC-VRBP1 has been observed to bind to HUVECs with high specificity. Furthermore, the successful optical/MR dual-modality imaging targeting angiogenesis in plaques has been obtained through applying VRBP1-UCNPs, confirming that it is a promising technique to detect unstable plaques in early-stage [119]. Unlike these studies, Wang et al. developed profilin-targeted magnetic nanoparticles, i.e. PC-NPs, as dual-modality molecular probes for murine atherosclerosis, and revealed their potential in characterizing VSMCs in plaques. Importantly, a good correlation between MRI signals and fluorescence intensities of imaging in Apoe-/- mice with PC-NPs injection was observed [120]. At last, Dai et al. submitted that the ROS-scavenging nanoparticles can mediate MR/fluorescence dual-modality imaging tracing of vulnerable plaques. It is worth pointing out that this is the only study to especially address the therapeutic potential of dual-modality nanoprobes in atherosclerosis, mechanistically through the downergulation of inflammation, apoptosis, and foam cell formation [48]. Taken together, optical/MR dual-model imaging nanoprobes have been under active investigation, and have gradually shown promising potential in early diagnosis and prevention of atherosclerosis.
Optical/ultrasound dual-modality imaging nanoprobes in atherosclerotic research
Among various diagnostic imaging techniques, ultrasound imaging has its own advantages for atherosclerotic plaques, for example, real-time monitoring capability, low cost, portability, and high safety [121, 122]. Thus, optical/ultrasound dual-modality imaging is a preferable integrated choice in this sense. Li and coworkers characterized osteopontin-targeted nanoparticles, i.e. COP-NPs, and showed that these nanoparticles are accumulated in vulnerable plaques, as demonstrated by both ultrasound and optical imaging. Of note, osteopontin-targeted nanoparticles have been verified as a good contrast agent in molecular imaging of foam cells and smooth muscle cells, which thereby can be an efficient tool to identify vulnerable plaques [123].
Photoacoustic/ultrasound dual-modality imaging nanoprobes in atherosclerotic research
For many years, an integrated intravascular imaging catheter has been successfully designed and developed, making it possible for the clinical realization of the hybrid intravascular ultrasound/intravascular photoacoustic (IVUS/IVPA) imaging system [124, 125]. Based on this novel imaging platform, Lin et al. constructed RGDfk peptide-targeted nanoprobes to study angiogenesis in atherosclerotic plaques, not only obtaining functional information of multiple components in plaques, such as neovascularization and lipid core, but also acquiring arterial structural information. In their study, an atherosclerotic rabbit model was utilized, and the imaging effects of photoacoustic/ultrasound dual-modality imaging nanoprobes on plaques were systematically evaluated in vivo [47]. From a clinical perspective, Yeager and coworkers successfully achieved ex vivo coronary artery plaque photoacoustic/ultrasound imaging in a patient undergoing autopsy by using silica-coated gold nanorods as contrast agents [126]. Even though there are few studies in this regard, hybrid intravascular molecular imaging technology is promising to provide a reliable platform for the early detection and intervention of atherosclerosis.
PET/CT dual-modality imaging nanoprobes in atherosclerotic research
Due to its high sensitivity and quantitative diagnosis, the integrated approach of PET/CT has been widely studied in preclinical and clinical atherosclerotic research among various integrated imaging modalities [127-129]. In this context, PET/CT imaging nanoprobes are mainly designed to target chemokine receptors, as exemplified by CCR5 which is mostly expressed in monocytes and neutrophils [130-132]. Luehmann et al. applied 64Cu-DOTA-DAPTA-comb and 64Cu-vMIP-II-comb nanoprobes in different studies, respectively, and found that plaque progression was in line with increased expression of chemokine receptors as well as elevated PET signals. These results together indicate the potential of PET/CT imaging nanoprobes in detecting plaque progression in C57BL/6 mice and/or Apoe-/- mice [130, 131]. In addition, the latest study from Detering and colleagues showed the application of CCR5-targeted peptide D-ala-peptide T-amide, i.e. DAPTA-comb nanoprobes, in PET/CT imaging of atherosclerotic plaques, and revealed physicochemical properties and targeting efficiency of DAPTA-comb. All three 64Cu-DAPTA-comb nanoprobes could visualize lesions by detecting CCR5 expression with high sensitivity and specificity [132]. Even though PET/CT imaging nanoprobes show broad prospects, their applications are also challenged, because that PET/CT imaging has some limitations, such as low soft-tissue contrast, and CT-related radiation exposure. For this reason, more PET/MR dual-modality imaging nanoprobes are under active investigation, which will be described emphatically in the following section.
PET/MR dual-modality imaging nanoprobes in atherosclerotic research
Nowadays, PET/MR dual-modality imaging has been extensively applied to study inflammation in atherosclerosis by mapping key molecular processes as well as functional parameters, as reviewed by Senders et al. recently [133]. Of note, high soft-tissue contrast can be achieved by PET combined with MR imaging. In clinical practice, Li et al. utilized [68Ga]Pentixafor PET/MR imaging to evaluate the expression of CXCR4 in human atherosclerotic lesions, highlighting CXCR4 as a surrogate marker for atherosclerosis [134, 135]. Similarly, several types of PET/MR dual-modality imaging nanoprobes have been experimentally investigated to improve the molecular diagnosis of atherosclerotic plaques, by targeting inflammatory macrophages, oxidized phospholipids, vascular calcifications, and angiogenesis.
Jarrett and colleagues synthesized 64Cu-labeled dextran sulfate-coated iron oxide nanoparticles, and thereby mapped macrophage distribution in lesion area using PET/MR imaging. Then, the enhanced contrast induced by these nanoprobes was observed at sites of vascular inflammation, but not in a normal vessel, in both PET and MR images [136]. In a similar vein, Tu et al. developed a kind of multifunctional PET/MRI nanoprobe, i.e. 64Cu-NOTA-IONP@MMP2c-PEG2K, MMP2cNPs, to evaluate the expression of MMP-2 in macrophage-rich vascular lesions. Interestingly, the excellent plaque-to-normal carotid artery contrast was obtained due to the rapid clearance of MMP2cNPs from the contralateral normal carotid artery. Moreover, iron was accumulated in atherosclerotic plaques, which was colocalized with MMP-2 in macrophages, as confirmed by histological analyses [137]. Taken together, the above-mentioned two kinds of nanoprobes are mainly assisting the molecular imaging of inflammatory macrophages in atherogenesis.
In contrast, Su et al. mainly looked into angiogenesis in a rabbit atherosclerotic model via using GEBP11 peptide-targeted magnetic iron oxide nanoparticles, showing good imaging properties, high stability, and little cytotoxicity as well as low immunogenicity, in the context of PET/MR dual-modality imaging [138]. Afterwards, Pellico and coworkers applied 68Ga iron oxide nano-radiomaterials for the targeted bioorthogonal molecular imaging, with their selective accumulation in atherosclerotic plaques in Apoe-/- mice [139]. More recently, Pellico et al. reported hydroxyapatite (HAP)-multitag as a PET contrast nano-tracer for the characterization of vascular calcifications in plaques, and revealed that HAP-multitag can support to achieve the early detection of plaques in 16-week-old Apoe-/- mice. Importantly, these imaging probes are capable of providing simultaneous signals in both modalities, i.e. PET/MRI [97]. In summary, PET/MR dual-modality imaging allows for non-invasive studies of atherosclerotic progression, and relevant molecular mechanisms as well.
Multi-modality imaging nanoprobes for early detection of plaques
Not only high-sensitivity imaging techniques but also specific targeting markers are needed for an ideal imaging platform to identify atherosclerotic plaques precisely and specifically. Therefore, developing multi-modality imaging nanoprobes is an appealing trend in the future, although there might be some technical challenges in constructing nanomaterials with tailored physical and biological properties. In fact, various multi-modality imaging has been applied to study tumorigenesis [140, 141], while few studies are available for atherosclerotic research. Dating back to 2008, Nahrendorf and colleagues performed multi-model imaging of macrophages in plaques by using a tri-modality reporter for PET, MRI, and fluorescence imaging, i.e. 64Cu-TNP, and observed a pronounced correlation between PET signals and CD68 expression. Of interest, they addressed the diagnostic capability of multi-modality nanoprobes in atherosclerosis and their clinical translatability as well [142]. Recently, Tong et al. developed a novel multimodal imaging agent, 5-HT-Fe3O4-Cy7 nanoparticles, for MRI, CT, and fluorescence imaging of vulnerable plaques by detecting active myeloperoxidase, displaying high sensitivity and specificity. The severity of inflammation and the activity of myeloperoxidase could be evaluated according to the accumulation of these nanoprobes in plaques [143]. With more relevant studies emerging, the potential of multi-modality imaging nanoprobes will be revealed, especially for early-stage plaques.
Molecular targets to formulate nanoprobes for atherosclerosis imaging
Considering the importance of molecular targets in determining the specificity of nanoprobes for plaque visualization, we would discuss currently-applied targets and some other potent candidates in this section, separately. In the meanwhile, relevant details and comparison of several well-investigated molecular targets, encompassing VCAM-1, OPN, MMP-2, CD47, CD44, MARCO, and VEGFR-2, etc., have been summarized in Table 4 as well as Figure 2.
Current plaque-relevant molecular targets that have been chosen for the design of nanoprobes, mainly include aptamers, peptides, antibodies, and others. In general, antibodies have high specificity and affinity, whereas high immunogenicity and poor stability. Additionally, high cost is also a disadvantage limiting antibodies' application. In contrast, peptides need relatively low production costs, with high specificity and safety but limited stability [144]. More recently, in terms of the high immunogenicity of antibodies as well as the low stability of peptides, aptamers with a couple of advantages offer a better type of ligands to construct new nanoprobes [145]. Thus, it is essential to choose the proper ligand pattern when designing nanoprobes for plaque imaging, although current studies have tested various ligands and made some progress to a certain extent.
On the other hand, as shown in Table 4, these targets are closely associated with different biological processes in atherogenesis, for example, inflammation, angiogenesis, vascular calcifications, and lipoprotein synthesis as well as clearance [28, 61, 77, 93, 102, 119]. Among them, inflammatory factors are the most common targets, such as IL-6, MARCO, CD36, CD47, and others. Nowadays, special attention is called for many other candidates which have not been tested yet but simultaneously involved in several pathological processes, as exemplified by some classical chemokines and atypical chemokines. CXCR4, as a known receptor for CXCL12 and macrophage migration inhibitory factors (MIFs), participates in atherogenic inflammation and lipid metabolism, which may support it as a promising candidate for the design of more specific nanoprobes to visualize plaques [146]. Therefore, exploring specific and selective molecular targets is a key step for the generation of novel nanoprobes. Based on this, it makes sense to further evaluate the imaging effects of plaques. In the future, more mechanistic investigation is still needed to discover new molecular targets of atherogenesis, supporting the construction of novel nanoprobes in this field.
Several well-investigated molecular targets in non-invasive imaging for atherosclerotic plaques
| Main processes | Molecular targets | Expression | Main biological functions | Formulated nanoprobes |
|---|---|---|---|---|
| Targeting inflammation | VCAM-1 | Immune cells and vascular endothelium | Cell adhesion | MR imaging nanoprobes |
| Osteopontin | Macrophages | Biomineralization, cell adhesion | Photoacoustic, MR imaging nanoprobes | |
| IL-6 | Immune cells, smooth muscle cells, endothelial cells, adipocytes | Acute phase | MR imaging nanoprobes | |
| MMP-2 | Fibroblasts | Angiogenesis, collagen degradation | Optical, photoacoustic imaging nanoprobes | |
| CD36 | Monocytes, endothelial cells, adipocytes, skeletal and cardiac muscle cells | Cell adhesion, lipid transport | Optical, photoacoustic imaging nanoprobes | |
| SR-AI | Macrophages, Hepatocytes, Adipocytes, Kupffer cells, Granulosa cells | Host-virus interaction | MR imaging nanoprobe | |
| CD47 | Monocytes/macrophages | Cell adhesion | Optical imaging nanoprobes | |
| MARCO | Macrophages, Kupffer cells | Immunity, innate immunity | Optical/MR imaging nanoprobes | |
| Targeting angiogenesis | VEGFR2 | HUVECs | Angiogenesis | Optical/MR imaging nanoprobes |
| NPR-C receptors | The endothelium of neovessels | Angiogenesis | PET imaging nanoprobes | |
| Targeting vascular calcifications | CD44 | Myeloid cells | Cell adhesion and migration | MR imaging nanoprobes |
| Targeting lipoproteins | HDL | Plasma | Reverse cholesterol transport | PET imaging nanoprobes |
Concluding remarks and future perspectives
In recent years, molecular imaging techniques that enable early monitoring of atherosclerotic plaques before clinical manifestation is an active area of research. Various single-, dual- as well as multi-modality imaging nanoprobes have been investigated and show promising prospects. However, optical/photoacoustic imaging nanoprobes have been only applied in cell lines in vitro and experimental animals in vivo, due to some limitations of the living biological imaging system, as exemplified by fluorescent nanoprobes. Especially, they are often enriched in the liver and difficult to metabolize, which leads to strong background signals and poor imaging quality. Although these shortcomings prevent their clinical application, they can still be utilized to study the pathological mechanisms of atherosclerosis, and evaluate the drug efficacy. In contrast to optical/photoacoustic nanoprobes, MR/PET nanoprobes have a higher likelihood to achieve preferable clinical translation once their specificity and selectivity are improved, without limitations of equipment.
In this regard, it is worth noting that there are several potential factors preventing these nanoprobes from entering the clinic, as implied by many original studies. First of all, targeting efficiency is a key issue. Owing to the limited specificity of current nanoprobes in detecting plaques, target-specific ligands are extremely needed to further improve the accuracy of the molecular diagnosis of early atherosclerosis. Nowadays, researchers have developed various types of nanoprobes coated with well-studied plaque-specific molecules, exerting tailored physical and biological properties. They on the one hand are beneficial to specific recognization of early-stage plaques, on the other hand, give additional insights into the molecular basis for atherogenesis. However, the presence of the same receptor in different cells still reduces the targeting capacity of the molecular probes, in turn limiting its clinical application and drawing more attention from researchers. Secondly, we have to consider the metabolic issues of nanomaterials when it comes to their application in patients. Recently, metabolizable near-infrared-II nanoprobes seem to be a better choice to overcome this drawback. Thirdly, the complexity and the required dosage of these nanoprobes for imaging need to be optimized to achieve the medical purpose, which is also one obvious limitation. Thus, these key factors should be taken into account when designing novel nanoprobes in the context of atherosclerosis.
In addition, dual- and multi-mode imaging combining the advantages of multiple single-mode imaging techniques become more popular, as single-modality imaging is not enough to provide accurate imaging information. For this reason, developing dual- and multi-modality nanoprobes and/or combining multiple molecular targets in one type of nanoprobe is the common trend in this field. Furthermore, rigorous validation of the signal origin and the probe fate would be guaranteed by the multi-modality capabilities of these nanomaterials. Undoubtedly, specific targets, metabolizable nanomaterials, and advanced equipment, are together to support the precise visualization of plaques. Of interest, cell membrane-coated nanotechnology has emerged as a promising therapeutic platform, which may facilitate the targeted delivery of anti-atherosclerosis drugs. In the future, multi-modality nanoprobes with multiple targets would strongly contribute to accurate diagnosis and efficient treatment of atherosclerosis, although their complexity may bring some technical difficulties.
In summary, this paper reviews different types of formulated nanoprobes applied for early detection and reverse of plaques, and especially highlights recent advances, many challenges as well as opportunities. Now clearly, with the development of metabolizable nanomaterials and the presence of more specific targets, the clinical value of nanoprobes with the critical property of specifically targeting early atherosclerosis has been increasing. Of special note, the new generation of more precise and efficient molecular nanoprobes also needs the close collaboration of cardiologists, chemists, clinical radiologists, radiobiologists, statisticians, molecular biologists, and immunologists. Taken all together, engineering nanoprobes, serving as precise diagnostic tools and promising therapeutic carriers, would provide more preferable choices for early diagnosis and prevention of atherosclerosis.
Abbreviations
CT: computed tomography; CTA: computed tomography angiography; MRI: magnetic resonance imaging; PET: positron emission tomography; AIE: aggregation induced emission; TNF: tumor necrosis factor; CD47: cluster of differentiation 47; PAI: photoacoustic imaging; IVPA: intravascular photoacoustic; IVUS: intravascular ultrasound; VASP: vulnerable atherosclerotic plaques; OPN: osteopontin; BSA: bovine serum albumin; SPIONs: superparamagnetic iron oxide nanoparticles; TF: tissue factor; CD36: cluster of differentiation 36; LDL: low-density lipoprotein; oxLDL: oxidized low-density lipoprotein; MRP: Myeloid-related protein; HLI: hind limb ischemia; CANF: 64Cu-labeled C-type atrial natriuretic factor; MMP-2: matrix metalloproteinase-2; ICG: indocyanine green; NPR-C: natriuretic peptide clearance receptor; COP-NPs: Cy5.5-anti-OPN-PEG-PLA-PFOB nanoparticles; VSMCs: vascular smooth muscle cells; MARCO: macrophage receptor with collagenous structure; BMMCs: bone marrow mononuclear cells; SR-A: scavenger receptor A; VEGFR2: vascular endothelial growth factor receptor 2; ROS: reactive oxygen species; HDL: high-density lipoprotein; AuNRs-Abs: gold nanorods conjugated with MMP-2 antibody; IL-6: interleukin-6; Anti-IL-6-USPIO: IL-6-targeted superparamagnetic iron oxide nanoparticles; 18F-FDG: (18)F-fluorodeoxyglucose; NIR: near-infrared; FR: folate receptor; HA: hyaluronan; HAP: hydroxyapatite; MMP2cNPs: 64Cu-NOTA-IONP@MMP2c-PEG2K; VCAM-1: vascular cellular adhesion molecule-1.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 82102103, 82203221, 81671724, 81971655, 81571716, 81471695, 62005150, 82027804, and U22A6008). Figures were created with BioRender.com.
Authors contributions
C. Zan conceived and designed the contents, structures, and layout of the review article with help from J. An, Z. Wu, and S. Li. The first draft of the manuscript was written by C. Zan and edited by J. An, Z. Wu, and S. Li. All authors commented on the manuscript draft. J. An, Z. Wu, and S. Li provided the funding.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20:589-610
2. Tokgözoğlu L, Libby P. The dawn of a new era of targeted lipid-lowering therapies. Eur Heart J. 2022;43:3198-208
3. Libby P, Hansson GK. From Focal Lipid Storage to Systemic Inflammation: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:1594-607
4. Speer T, Dimmeler S, Schunk SJ, Fliser D, Ridker PM. Targeting innate immunity-driven inflammation in CKD and cardiovascular disease. Nat Rev Nephrol. 2022
5. Poredoš P, Cífková R, Marie Maier JA, Nemcsik J, Šabovič M, Jug B. et al. Preclinical atherosclerosis and cardiovascular events: Do we have a consensus about the role of preclinical atherosclerosis in the prediction of cardiovascular events? Atherosclerosis. 2022;348:25-35
6. Jacobs DRJ, Woo JG, Sinaiko AR, Daniels SR, Ikonen J, Juonala M. et al. Childhood Cardiovascular Risk Factors and Adult Cardiovascular Events. N Engl J Med. 2022;386:1877-88
7. Burggraaf B, van Breukelen-van der Stoep DF, de Vries MA, Klop B, Liem AH, van de Geijn GM. et al. Effect of a treat-to-target intervention of cardiovascular risk factors on subclinical and clinical atherosclerosis in rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2019;78:335-41
8. Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524-33
9. Wang Y, Zhang K, Li T, Maruf A, Qin X, Luo L. et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics. 2021;11:164-80
10. Botts SR, Fish JE, Howe KL. Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment. Front Pharmacol. 2021;12:787541
11. Al Rifai M, Ahmed AI, Alahdab F, Al-Mallah MH. Clinical utility of coronary artery computed tomography angiography- What we know and What's new? Prog Cardiovasc Dis. 2022;75:12-20
12. Cho H, Kang SJ, Min HS, Lee JG, Kim WJ, Kang SH. et al. Intravascular ultrasound-based deep learning for plaque characterization in coronary artery disease. Atherosclerosis. 2021;324:69-75
13. Chen W, Li R, Yin K, Liang J, Li H, Chen X. et al. Clinical feasibility of using effective atomic number maps derived from non-contrast spectral computed tomography to identify non-calcified atherosclerotic plaques: a preliminary study. Quant Imaging Med Surg. 2022;12:2280-7
14. Onnis C, Cadeddu Dessalvi C, Cademartiri F, Muscogiuri G, Angius S, Contini F. et al. Quantitative and qualitative features of carotid and coronary atherosclerotic plaque among men and women. Front Cardiovasc Med. 2022;9:970438
15. van den Hoogen IJ, Schultz J, Kuneman JH, de Graaf MA, Kamperidis V, Broersen A. et al. Detailed behaviour of endothelial wall shear stress across coronary lesions from non-invasive imaging with coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2022;23:1708-16
16. Jakubauskas D, Jansen M, Lyngsø J, Cheng Y, Pedersen JS, Cárdenas M. Toward reliable low-density lipoprotein ultrastructure prediction in clinical conditions: A small-angle X-ray scattering study on individuals with normal and high triglyceride serum levels. Nanomedicine. 2021;31:102318
17. Tsimikas S, Narula J. Lipoprotein(a) and CT Angiography: Novel Insights Into High-Risk Plaque Progression. J Am Coll Cardiol. 2022;79:234-7
18. Neisius U, Gona PN, Oyama-Manabe N, Chuang ML, O'Donnell CJ, Manning WJ. et al. Relation of MRI Aortic Wall Area and Plaque to Incident Cardiovascular Events: The Framingham Heart Study. Radiology. 2022;304:542-50
19. Zhang L, Li L, Feng G, Fan T, Jiang H, Wang Z. Advances in CT Techniques in Vascular Calcification. Front Cardiovasc Med. 2021;8:716822
20. Zhou P, Wang Y, Sun J, Yu Y, Mossa-Basha M, Zhu C. Assessment of Therapeutic Response to Statin Therapy in Patients With Intracranial or Extracranial Carotid Atherosclerosis by Vessel Wall MRI: A Systematic Review and Updated Meta-Analysis. Front Cardiovasc Med. 2021;8:742935
21. Wilkins JT, Gidding SS, Robinson JG. Can atherosclerosis be cured? Curr Opin Lipidol. 2019;30:477-84
22. Zambon A, Mello E Silva A, Farnier M. The burden of cholesterol accumulation through the lifespan: why pharmacological intervention should start earlier to go further? Eur Heart J Cardiovasc Pharmacother. 2021;7:435-41
23. Lin J, Li H, Guo J, Xu Y, Li H, Yan J. et al. Potential of fluorescent nanoprobe in diagnosis and treatment of Alzheimer's disease. Nanomedicine (Lond). 2022;17:1191-211
24. Cheng K, Cheng Z. Near infrared receptor-targeted nanoprobes for early diagnosis of cancers. Curr Med Chem. 2012;19:4767-85
25. Yang H, Yu Z, Ji S, Huo Q, Yan J, Gao Y. et al. Targeting bone microenvironments for treatment and early detection of cancer bone metastatic niches. J Control Release. 2022;341:443-56
26. He H, Zhang X, Du L, Ye M, Lu Y, Xue J. et al. Molecular imaging nanoprobes for theranostic applications. Adv Drug Deliv Rev. 2022 186
27. Xu M, Mao C, Chen H, Liu L, Wang Y, Hussain A. et al. Osteopontin targeted theranostic nanoprobes for laser-induced synergistic regression of vulnerable atherosclerotic plaques. Acta Pharm Sin B. 2022;12:2014-28
28. Wang K, Gao H, Zhang Y, Yan H, Si J, Mi X. et al. Highly Bright AIE Nanoparticles by Regulating the Substituent of Rhodanine for Precise Early Detection of Atherosclerosis and Drug Screening. Adv Mater. 2022;34:e2106994
29. Dai T, He W, Tu S, Han J, Yuan B, Yao C. et al. Black TiO2 nanoprobe-mediated mild phototherapy reduces intracellular lipid levels in atherosclerotic foam cells via cholesterol regulation pathways instead of apoptosis. Bioact Mater. 2022;17:18-28
30. Vermeulen I, Isin EM, Barton P, Cillero-Pastor B, Heeren RMA. Multimodal molecular imaging in drug discovery and development. Drug Discov Today. 2022;27:2086-99
31. van de Donk PP, Oosting SF, Knapen DG, van der Wekken AJ, Brouwers AH, Lub-de Hooge MN. et al. Molecular imaging to support cancer immunotherapy. J Immunother Cancer. 2022;10:e004949
32. Li X, Wang R, Zhang Y, Han S, Gan Y, Liang Q. et al. Molecular imaging of tumor-associated macrophages in cancer immunotherapy. Ther Adv Med Oncol. 2022;14:17588359221076194
33. Wu L, Zou H, Wang H, Zhang S, Liu S, Jiang Y. et al. Update on the development of molecular imaging and nanomedicine in China: Optical imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13:e1660
34. Liu Y, Li Y, Koo S, Sun Y, Liu Y, Liu X. et al. Versatile Types of Inorganic/Organic NIR-IIa/IIb Fluorophores: From Strategic Design toward Molecular Imaging and Theranostics. Chem Rev. 2022;122:209-68
35. MacRitchie N, Noonan J, Guzik TJ, Maffia P. Molecular imaging of cardiovascular inflammation. Br J Pharmacol. 2021;178:4216-45
36. Wang X, Ziegler M, McFadyen JD, Peter K. Molecular imaging of arterial and venous thrombosis. Br J Pharmacol. 2021;178:4246-69
37. Quillard T, Croce K, Jaffer FA, Weissleder R, Libby P. Molecular imaging of macrophage protease activity in cardiovascular inflammation in vivo. Thromb Haemost. 2011;105:828-36
38. Qiao R, Huang X, Qin Y, Li Y, Davis TP, Hagemeyer CE. et al. Recent advances in molecular imaging of atherosclerotic plaques and thrombosis. Nanoscale. 2020;12:8040-64
39. Wang J, Li L, Li Y, Liu L, Li J, Li X. et al. PSMA1-mediated ultrasmall gold nanoparticles facilitate tumor targeting and MR/CT/NIRF multimodal detection of early-stage prostate cancer. Nanomedicine. 2023;47:102617
40. Hariri A, Lemaster J, Wang J, Jeevarathinam AS, Chao DL, Jokerst JV. The characterization of an economic and portable LED-based photoacoustic imaging system to facilitate molecular imaging. Photoacoustics. 2017;9:10-20
41. Tu S, He W, Han J, Wu A, Ren W. Advances in imaging and treatment of atherosclerosis based on organic nanoparticles. APL Bioeng. 2022;6:041501
42. Zhang M, Xie Z, Long H, Ren K, Hou L, Wang Y. et al. Current advances in the imaging of atherosclerotic vulnerable plaque using nanoparticles. Mater Today Bio. 2022;14:100236
43. Jiao M, Zhang P, Meng J, Li Y, Liu C, Luo X. et al. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomater Sci. 2018;6:726-45
44. Bogart LK, Pourroy G, Murphy CJ, Puntes V, Pellegrino T, Rosenblum D. et al. Nanoparticles for imaging, sensing, and therapeutic intervention. ACS Nano. 2014;8:3107-22
45. Yao Y, Li B, Yin C, Cong F, Ma GS, Liu NF. et al. A Folate-Conjugated Dual-Modal Fluorescent Magnetic Resonance Imaging Contrast Agent that Targets Activated Macrophages In Vitro and In Vivo. J Biomed Nanotechnol. 2016;12:2161-71
46. Wang Y, Zhang Y, Wang Z, Zhang J, Qiao RR, Xu M. et al. Optical/MRI dual-modality imaging of M1 macrophage polarization in atherosclerotic plaque with MARCO-targeted upconversion luminescence probe. Biomaterials. 2019;219:119378
47. Lin L, Xie Z, Xu M, Wang Y, Li S, Yang N. et al. IVUS\IVPA hybrid intravascular molecular imaging of angiogenesis in atherosclerotic plaques via RGDfk peptide-targeted nanoprobes. Photoacoustics. 2021;22:100262
48. Dai Y, Sha X, Song X, Zhang X, Xing M, Liu S. et al. Targeted Therapy of Atherosclerosis Vulnerable Plaque by ROS-Scavenging Nanoparticles and MR/Fluorescence Dual-Modality Imaging Tracing. Int J Nanomedicine. 2022;17:5413-29
49. Li Y, Zeng S, Hao J. Non-Invasive Optical Guided Tumor Metastasis/Vessel Imaging by Using Lanthanide Nanoprobe with Enhanced Down-Shifting Emission beyond 1500 nm. ACS Nano. 2019;13:248-59
50. Xu G, Yan Q, Lv X, Zhu Y, Xin K, Shi B. et al. Imaging of Colorectal Cancers Using Activatable Nanoprobes with Second Near-Infrared Window Emission. Angew Chem Int Ed Engl. 2018;57:3626-30
51. Wang F, Qu L, Ren F, Baghdasaryan A, Jiang Y, Hsu R. et al. High-precision tumor resection down to few-cell level guided by NIR-IIb molecular fluorescence imaging. Proc Natl Acad Sci U S A. 2022;119:e2123111119
52. Douma K, Megens RT, van Zandvoort MA. Optical molecular imaging of atherosclerosis using nanoparticles: shedding new light on the darkness. Wiley Interdiscip Rev Nanomed Nanobiotechnol. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:376-88
53. Chen W, Schilperoort M, Cao Y, Shi J, Tabas I, Tao W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol. 2022;19:228-49
54. Almas T, Haider R, Malik J, Mehmood A, Alvi A, Naz H. et al. Nanotechnology in interventional cardiology: A state-of-the-art review. Int J Cardiol Heart Vasc. 2022;43:101149
55. Jarr KU, Ye J, Kojima Y, Ye Z, Gao H, Schmid S. et al. The pleiotropic benefits of statins include the ability to reduce CD47 and amplify the effect of pro-efferocytic therapies in atherosclerosis. Nat Cardiovasc Res. 2022;1:253-62
56. Ye ZM, Yang S, Xia YP, Hu RT, Chen S, Li BW. et al. LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis. 2019;10:138
57. Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL. et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86-90
58. Flores AM, Hosseini-Nassab N, Jarr KU, Ye J, Zhu X, Wirka R. et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat Nanotechnol. 2020;15:154-61
59. Chen Y, Yang M, Huang W, Chen W, Zhao Y, Schulte ML. et al. Mitochondrial Metabolic Reprogramming by CD36 Signaling Drives Macrophage Inflammatory Responses. Circ Res. 2019;125:1087-102
60. Chen Y, Zhang J, Cui W, Silverstein RL. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J Exp Med. 2022;219:e20211314
61. Sun Y, Gao W, Liu Z, Huazhen, Yang H, Cao W. Luminescence-Resonance-Energy-Transfer-Based Luminescence Nanoprobe for In Situ Imaging of CD36 Activation and CD36-oxLDL Binding in Atherogenesis. Analytical chemistry. 2019;91:9770-6
62. Momi S, Falcinelli E, Petito E, Ciarrocca Taranta G, Ossoli A, Gresele P. Matrix metalloproteinase-2 on activated platelets triggers endothelial PAR-1 initiating atherosclerosis. Eur Heart J. 2022;43:504-14
63. Zhang S, Zhu X, Li G. E2F1/SNHG7/miR-186-5p/MMP2 axis modulates the proliferation and migration of vascular endothelial cell in atherosclerosis. Life Sci. 2020;257:118013
64. Park HJ, Kim MK, Kim Y, Bae SS, Kim HJ, Bae SK. et al. Gastrin-releasing peptide promotes the migration of vascular smooth muscle cells through upregulation of matrix metalloproteinase-2 and -9. BMB Rep. 2017;50:628-33
65. Han ME, Baek S, Kim HJ, Lee JH, Ryu SH, Oh SO. Development of an aptamer-conjugated fluorescent nanoprobe for MMP2. Nanoscale Res Lett. 2014;9:104
66. Chen JA, Guo W, Wang Z, Sun N, Gu X. In Vivo Imaging of Senescent Vascular Cells in Atherosclerotic Mice Using a β-Galactosidase-Activatable Nanoprobe. Analytical Chemistry. 2020;92:12613-21
67. Lozano-Torres B, Galiana I, Rovira M, Garrido E, Chaib S, Bernardos A. et al. An OFF-ON Two-Photon Fluorescent Probe for Tracking Cell Senescence in Vivo. J Am Chem Soc. 2017;139:8808-11
68. Zhang J, Li C, Dutta C, Fang M, Zhang S, Tiwari A. et al. A novel near-infrared fluorescent probe for sensitive detection of β-galactosidase in living cells. Anal Chim Acta. 2017;968:97-104
69. Wang Q, Wang Y, Liu S, Sha X, Song X, Dai Y. et al. Theranostic nanoplatform to target macrophages enables the inhibition of atherosclerosis progression and fluorescence imaging of plaque in ApoE(-/-) mice. J Nanobiotechnology. 2021;19:222
70. Choi SSS, Mandelis A. Review of the state of the art in cardiovascular endoscopy imaging of atherosclerosis using photoacoustic techniques with pulsed and continuous-wave optical excitations. J Biomed Opt. 2019;24:1-15
71. Gifani M, Eddins DJ, Kosuge H, Zhang Y, Paluri SLA, Larson T. et al. Ultra-selective carbon nanotubes for photoacoustic imaging of inflamed atherosclerotic plaques. Adv Funct Mater. 2021;31:2101005
72. Upputuri PK, Pramanik M. Recent advances in photoacoustic contrast agents for in vivo imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:e1618
73. Jansen K, van Soest G, van der Steen AF. Intravascular photoacoustic imaging: a new tool for vulnerable plaque identification. Ultrasound Med Biol. 2014;40:1037-48
74. Iskander-Rizk S, Wu M, Springeling G, van Beusekom HMM, Mastik F, Te Lintel Hekkert M. et al. In vivo intravascular photoacoustic imaging of plaque lipid in coronary atherosclerosis. EuroIntervention. 2019;15:452-6
75. Bourantas CV, Jaffer FA, Gijsen FJ, van Soest G, Madden SP, Courtney BK. et al. Hybrid intravascular imaging: recent advances, technical considerations, and current applications in the study of plaque pathophysiology. Eur Heart J. 2017;38:400-12
76. Wu C, Zhang Y, Li Z, Li C, Wang Q. A novel photoacoustic nanoprobe of ICG@PEG-Ag2S for atherosclerosis targeting and imaging in vivo. Nanoscale. 2016;8:12531-9
77. Qin H, Zhao Y, Zhang J, Pan X, Yang S, Xing D. Inflammation-targeted gold nanorods for intravascular photoacoustic imaging detection of matrix metalloproteinase-2 (MMP2) in atherosclerotic plaques. Nanomedicine. 2016;12:1765-74
78. Gao W, Li X, Liu Z, Fu W, Sun Y, Cao W. et al. A Redox-Responsive Self-Assembled Nanoprobe for Photoacoustic Inflammation Imaging to Assess Atherosclerotic Plaque Vulnerability. Anal Chem. 2019;91:1150-6
79. Ge X, Cui H, Kong J, Lu SY, Zhan R, Gao J. et al. A Non-Invasive Nanoprobe for In Vivo Photoacoustic Imaging of Vulnerable Atherosclerotic Plaque. Adv Mater. 2020;32:e2000037
80. Xie Z, Yang Y, He Y, Shu C, Chen D, Zhang J. et al. In vivo assessment of inflammation in carotid atherosclerosis by noninvasive photoacoustic imaging Theranostics. 2020; 10: 4694-704.
81. Evans NR, Tarkin JM, Le EP, Sriranjan RS, Corovic A, Warburton EA. et al. Integrated cardiovascular assessment of atherosclerosis using PET/MRI. Br J Radiol. 2020;93:20190921
82. Wüst RCI, Calcagno C, Daal MRR, Nederveen AJ, Coolen BF, Strijkers GJ. Emerging Magnetic Resonance Imaging Techniques for Atherosclerosis Imaging. Arterioscler Thromb Vasc Biol. 2019;39:841-9
83. Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M. et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453-8
84. Segers FME, Ruder AV, Westra MM, Lammers T, Dadfar SM, Roemhild K. et al. MRI Contrast-enhancement with superparamagnetic iron oxide nanoparticles amplify macrophage foam cell apoptosis in human and murine atherosclerosis. Cardiovasc Res. 2022: cvac032.
85. Smith BR, Heverhagen J, Knopp M, Schmalbrock P, Shapiro J, Shiomi M. et al. Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI). Biomed Microdevices. 2007;9:719-27
86. Wei Q, Wang J, Shi W, Zhang B, Hu Y. Improved in vivo detection of atherosclerotic plaques with a tissue factor-targeting magnetic nanoprobe. Acta Biomaterialia. 2019;90:324-36
87. Mo H, Fu C, Wu Z, Liu P, Wen Z, Hong Q. et al. IL-6-targeted ultrasmall superparamagnetic iron oxide nanoparticles for optimized MRI detection of atherosclerotic vulnerable plaques in rabbits. RSC Adv. 2020;10:15346-53
88. Huang X, Lin C, Luo C, Guo Y, Li J, Wang Y. et al. Fe3O4@M nanoparticles for MRI-targeted detection in the early lesions of atherosclerosis. Nanomedicine. 2021;33:102348
89. Grover SP, Mackman N. Tissue factor in atherosclerosis and atherothrombosis. Atherosclerosis. 2020;307:80-6
90. Ionita MG, Vink A, Dijke IE, Laman JD, Peeters W, van der Kraak PH. et al. High levels of myeloid-related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2009;29:1220-7
91. Maiseyeu A, Badgeley MA, Kampfrath T, Mihai G, Deiuliis JA, Liu C. et al. In vivo targeting of inflammation-associated myeloid-related protein 8/14 via gadolinium immunonanoparticles. Arterioscler Thromb Vasc Biol. 2012;32:962-70
92. Hossaini Nasr S, Tonson A, El-Dakdouki MH, Zhu DC, Agnew D, Wiseman R. et al. Effects of Nanoprobe Morphology on Cellular Binding and Inflammatory Responses: Hyaluronan-Conjugated Magnetic Nanoworms for Magnetic Resonance Imaging of Atherosclerotic Plaques. ACS Appl Mater Interfaces. 2018;10:11495-507
93. Chen Q, Guo C, Zhou X, Su Y, Guo H, Cao M. et al. N-acetylneuraminic acid and chondroitin sulfate modified nanomicelles with ROS-sensitive H2S donor via targeting E-selectin receptor and CD44 receptor for the efficient therapy of atherosclerosis. Int J Biol Macromol. 2022;211:259-70
94. Krolikoski M, Monslow J, Puré E. The CD44-HA axis and inflammation in atherosclerosis: A temporal perspective. Matrix Biol. 2019;78-79:201-18
95. Tang T, Tu C, Chow SY, Leung KH, Du S, Louie AY. Quantitative assessment of binding affinities for nanoparticles targeted to vulnerable plaque. Bioconjug Chem. 2015;26:1086-94
96. Sriranjan RS, Tarkin JM, Evans NR, Le EPV, Chowdhury MM, Rudd JHF. Atherosclerosis imaging using PET: Insights and applications. Br J Pharmacol. 2021;178:2186-203
97. Pellico J, Fernández-Barahona I, Ruiz-Cabello J, Gutiérrez L, Muñoz-Hernando M, Sánchez-Guisado MJ. et al. HAP-Multitag, a PET and Positive MRI Contrast Nanotracer for the Longitudinal Characterization of Vascular Calcifications in Atherosclerosis. ACS Appl Mater Interfaces. 2021 13
98. Florea A, Sigl JP, Morgenroth A, Vogg A, Sahnoun S, Winz OH. et al. Sodium [18F]Fluoride PET Can Efficiently Monitor In Vivo Atherosclerotic Plaque Calcification Progression and Treatment. Cells. 2021;10:275
99. Perrotta P, Emini Veseli B, Van der Veken B, Roth L, Martinet W, De Meyer GRY. Pharmacological strategies to inhibit intra-plaque angiogenesis in atherosclerosis. Vascul Pharmacol. 2019;112:72-8
100. Liu Y, Pressly ED, Abendschein DR, Hawker CJ, Woodard GE, Woodard PK. et al. Targeting angiogenesis using a C-type atrial natriuretic factor-conjugated nanoprobe and PET. J Nucl Med. 2011;52:1956-63
101. Liu Y, Luehmann HP, Detering L, Pressly ED, McGrath AJ, Sultan D. et al. Assessment of Targeted Nanoparticle Assemblies for Atherosclerosis Imaging with Positron Emission Tomography and Potential for Clinical Translation. ACS Appl Mater Interfaces. 2019;11:15316-21
102. Pérez-Medina C, Binderup T, Lobatto ME, Tang J, Calcagno C, Giesen L. et al. In Vivo PET Imaging of HDL in Multiple Atherosclerosis Models. JACC Cardiovasc Imaging. 2016;9:950-61
103. Woodard PK, Liu Y, Pressly ED, Luehmann HP, Detering L, Sultan DE. et al. Design and Modular Construction of a Polymeric Nanoparticle for Targeted Atherosclerosis Positron Emission Tomography Imaging: A Story of 25% (64)Cu-CANF-Comb. Pharm Res. 2016;33:2400-10
104. Blankstein R, Di Carli MF. Integration of coronary anatomy and myocardial perfusion imaging. Nat Rev Cardiol. 2010;7:226-36
105. Li J, Montarello NJ, Hoogendoorn A, Verjans JW, Bursill CA, Peter K. et al. Multimodality Intravascular Imaging of High-Risk Coronary Plaque. JACC Cardiovasc Imaging. 2022;15:145-59
106. Daghem M, Bing R, Fayad ZA, Dweck MR. Noninvasive Imaging to Assess Atherosclerotic Plaque Composition and Disease Activity: Coronary and Carotid Applications. JACC Cardiovasc Imaging. 2020;13:1055-68
107. Fujimoto K, Angelone LM, Lucano E, Rajan SS, Iacono MI. Radio-Frequency Safety Assessment of Stents in Blood Vessels During Magnetic Resonance Imaging. Front Physiol. 2018;9:1439
108. de Havenon A, Haynor DR, Tirschwell DL, Majersik JJ, Smith G, Cohen W. et al. Association of Collateral Blood Vessels Detected by Arterial Spin Labeling Magnetic Resonance Imaging With Neurological Outcome After Ischemic Stroke. JAMA Neurol. 2017;74:453-8
109. Li Z, Tang H, Tu Y. Molecular and Nonmolecular Imaging of Macrophages in Atherosclerosis. Front Cardiovasc Med. 2021;8:670639
110. Baria E, Nesi G, Santi R, Maio V, Massi D, Pratesi C. et al. Improved label-free diagnostics and pathological assessment of atherosclerotic plaques through nonlinear microscopy. J Biophotonics. 2018;11:e201800106
111. Qiao H, Wang Y, Zhang R, Gao Q, Liang X, Gao L. et al. MRI/optical dual-modality imaging of vulnerable atherosclerotic plaque with an osteopontin-targeted probe based on Fe3O4 nanoparticles. Biomaterials. 2017;112:336-45
112. Shi X, Wu H, Liu Y, Huang H, Liu L, Yang Y. et al. Inhibiting vascular smooth muscle cell proliferation mediated by osteopontin via regulating gut microbial lipopolysaccharide: A novel mechanism for paeonol in atherosclerosis treatment. Front Pharmacol. 2022;13:936677
113. Yu W, Xiao L, Que Y, Li S, Chen L, Hu P. et al. Smooth muscle NADPH oxidase 4 promotes angiotensin II-induced aortic aneurysm and atherosclerosis by regulating osteopontin. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165912
114. Qiao R, Qiao H, Zhang Y, Wang Y, Chi C, Tian J. et al. Molecular Imaging of Vulnerable Atherosclerotic Plaques in Vivo with Osteopontin-Specific Upconversion Nanoprobes. ACS Nano. 2017;11:1816-25
115. Furusho Y, Miyata M, Matsuyama T, Nagai T, Li H, Akasaki Y. et al. Novel Therapy for Atherosclerosis Using Recombinant Immunotoxin Against Folate Receptor β-Expressing Macrophages. J Am Heart Assoc. 2012;1:e003079
116. Wen X, Shi C, Yang L, Zeng X, Lin X, Huang J. et al. A radioiodinated FR-β-targeted tracer with improved pharmacokinetics through modification with an albumin binder for imaging of macrophages in AS and NAFL. Eur J Nucl Med Mol Imaging. 2022;49:503-16
117. Poh S, Putt KS, Low PS. Folate-Targeted Dendrimers Selectively Accumulate at Sites of Inflammation in Mouse Models of Ulcerative Colitis and Atherosclerosis. Biomacromolecules. 2017;18:3082-8
118. Wang J, Wu M, Chang J, Li L, Guo Q, Hao J. et al. Scavenger receptor-AI-targeted ultrasmall gold nanoclusters facilitate in vivo MR and ex vivo fluorescence dual-modality visualization of vulnerable atherosclerotic plaques. Nanomedicine. 2019;19:81-94
119. Fang Y, Yang R, Hou Y, Wang Y, Yang N, Xu M. et al. Dual-modality Imaging of Angiogenesis in Unstable Atherosclerotic Plaques with VEGFR2-Targeted Upconversion Nanoprobes in vivo. Mol Imaging Biol. 2022;24:721-31
120. Wang Y, Chen J, Yang B, Qiao H, Gao L, Su T. et al. In vivo MR and Fluorescence Dual-modality Imaging of Atherosclerosis Characteristics in Mice Using Profilin-1 Targeted Magnetic Nanoparticles. Theranostics. 2016;6:272-86
121. Zhang L, Lyu Q, Zhou W, Li X, Ni Q, Jiang S. et al. High systemic immune-inflammation index is associated with carotid plaque vulnerability: New findings based on carotid ultrasound imaging in patients with acute ischemic stroke. Front Neurol. 2022;13:959531
122. Lyu Q, Tian X, Ding Y, Yan Y, Huang Y, Zhou P. et al. Evaluation of Carotid Plaque Rupture and Neovascularization by Contrast-Enhanced Ultrasound Imaging: an Exploratory Study Based on Histopathology. Transl Stroke Res. 2021;12:49-56
123. Li S, Gou T, Wang Q, Chen M, Chen Z, Xu M. et al. Ultrasound/Optical Dual-Modality Imaging for Evaluation of Vulnerable Atherosclerotic Plaques with Osteopontin Targeted Nanoparticles. Macromol Biosci. 2020;20:e1900279
124. Karpiouk AB, Wang B, Emelianov SY. Development of a catheter for combined intravascular ultrasound and photoacoustic imaging. Rev Sci Instrum. 2010;81:014901
125. Li Y, Gong X, Liu C, Lin R, Hau W, Bai X. et al. High-speed intravascular spectroscopic photoacoustic imaging at 1000 A-lines per second with a 0.9-mm diameter catheter. J Biomed Opt. 2015;20:065006
126. Yeager D, Chen YS, Litovsky S, Emelianov S. Intravascular photoacoustics for image-guidance and temperature monitoring during plasmonic photothermal therapy of atherosclerotic plaques: a feasibility study. Theranostics. 2013;4:36-46
127. Creager MD, Hohl T, Hutcheson JD, Moss AJ, Schlotter F, Blaser MC. et al. 18F-Fluoride Signal Amplification Identifies Microcalcifications Associated With Atherosclerotic Plaque Instability in Positron Emission Tomography/Computed Tomography Images. Circ Cardiovasc Imaging. 2019;12:e007835
128. Kircher M, Tran-Gia J, Kemmer L, Zhang X, Schirbel A, Werner RA. et al. Imaging Inflammation in Atherosclerosis with CXCR4-Directed 68Ga-Pentixafor PET/CT: Correlation with 18F-FDG PET/CT. J Nucl Med. 2020;61:751-6
129. Patel NH, Osborne MT, Teague H, Parel P, Svirydava M, Sorokin AV. et al. Heightened splenic and bone marrow uptake of 18F-FDG PET/CT is associated with systemic inflammation and subclinical atherosclerosis by CCTA in psoriasis: An observational study. Atherosclerosis. 2021;339:20-6
130. Luehmann HP, Pressly ED, Detering L, Wang C, Pierce R, Woodard PK. et al. PET/CT imaging of chemokine receptor CCR5 in vascular injury model using targeted nanoparticle. J Nucl Med. 2014;55:629-34
131. Luehmann HP, Detering L, Fors BP, Pressly ED, Woodard PK, Randolph GJ. et al. PET/CT Imaging of Chemokine Receptors in Inflammatory Atherosclerosis Using Targeted Nanoparticles. J Nucl Med. 2016;57:1124-9
132. Detering L, Abdilla A, Luehmann HP, Williams JW, Huang LH, Sultan D. et al. CC Chemokine Receptor 5 Targeted Nanoparticles Imaging the Progression and Regression of Atherosclerosis Using Positron Emission Tomography/Computed Tomography. Mol Pharm. 2021;18:1386-96
133. Senders ML, Calcagno C, Tawakol A, Nahrendorf M, Mulder WJM, Fayad ZA. PET/MR imaging of inflammation in atherosclerosis. Nat Biomed Eng. 2022
134. Li X, Heber D, Leike T, Beitzke D, Lu X, Zhang X. et al. [68Ga]Pentixafor-PET/MRI for the detection of Chemokine receptor 4 expression in atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2018;45:558-66
135. Li X, Yu W, Wollenweber T, Lu X, Wei Y, Beitzke D. et al. [68Ga]Pentixafor PET/MR imaging of chemokine receptor 4 expression in the human carotid artery. Eur J Nucl Med Mol Imaging. 2019;46:1616-25
136. Jarrett BR, Correa C, Ma KL, Louie AY. In vivo mapping of vascular inflammation using multimodal imaging. PLoS One. 2010;5:e13254
137. Tu Y, Ma X, Chen H, Fan Y, Jiang L, Zhang R. et al. Molecular Imaging of Matrix Metalloproteinase-2 in Atherosclerosis Using a Smart Multifunctional PET/MRI Nanoparticle. Int J Nanomedicine. 2022;17:6773-89
138. Su T, Wang YB, Han D, Wang J, Qi S, Gao L. et al. Multimodality Imaging of Angiogenesis in a Rabbit Atherosclerotic Model by GEBP11 Peptide Targeted Nanoparticles. Theranostics. 2017;7:4791-804
139. Pellico J, Fernández-Barahona I, Benito M, Gaitán-Simón Á, Gutiérrez L, Ruiz-Cabello J. et al. Unambiguous detection of atherosclerosis using bioorthogonal nanomaterials. Nanomedicine. 2019;17:26-35
140. Shi B, Zhang B, Zhang Y, Gu Y, Zheng C, Yan J. et al. Multifunctional gap-enhanced Raman tags for preoperative and intraoperative cancer imaging. Acta Biomaterialia. 2020;104:210-20
141. Sun H, Zhang B, Jiang X, Liu H, Deng S, Li Z. et al. Radiolabeled ultra-small Fe3O4 nanoprobes for tumor-targeted multimodal imaging. Nanomedicine (Lond). 2019;14:5-17
142. Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E. et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379-87
143. Tong W, Hui H, Shang W, Zhang Y, Tian F, Ma Q. et al. Highly sensitive magnetic particle imaging of vulnerable atherosclerotic plaque with active myeloperoxidase-targeted nanoparticles. Theranostics. 2021;11:506-21
144. Sinitski D, Kontos C, Krammer C, Asare Y, Kapurniotu A, Bernhagen J. Macrophage Migration Inhibitory Factor (MIF)-Based Therapeutic Concepts in Atherosclerosis and Inflammation. Thromb Haemost. 2019;119:553-66
145. Zuo Y, Xia Y, Lu W, Li Y, Xiao Y, Gao S. et al. A multifunctional black phosphorus nanosheet-based immunomagnetic bio-interface for heterogeneous circulating tumor cell capture and simultaneous self-identification in gastric cancer patients. Nanoscale. 2023;15:3872-83
146. El Bounkari O, Zan C, Wagner J, Bugar E, Bourilhon P, Kontos C. et al. MIF-2/D-DT is an atypical atherogenic chemokine that promotes advanced atherosclerosis and hepatic lipogenesis. bioRxiv. 2021
Author contact
![]() Corresponding authors: Prof. Zhifang Wu, E-mail: wuzhifang01com. Prof. Sijin Li, E-mail: lisjnm123com.
Corresponding authors: Prof. Zhifang Wu, E-mail: wuzhifang01com. Prof. Sijin Li, E-mail: lisjnm123com.

 Global reach, higher impact
Global reach, higher impact