ISSN: 2206-7418
Nanotheranostics 2023; 7(3):236-257. doi:10.7150/ntno.77564 This issue Cite
Review
Natural and synthetic nanovectors for cancer therapy
1. Department of Biochemistry, Faculty of Science, Ege University, Izmir 35040, Turkey
2. Institute of Molecular Biology & Biotechnologies, Ministry of Science and Education Republic of Azerbaijan, 11 Izzat Nabiyev, AZ1073 Baku, Azerbaijan
3. Department of Chemistry and Pharmacy, Physical Chemistry I and ICMM, Friedrich-Alexander University of Erlangen─Nuremberg, Egerlandstrasse 3, D-91058 Erlangen, Germany
4. Department of Biomedical Sciences, University of Lausanne, Rue du Bugnon 7, 1005 Lausanne, Switzerland
5. Swiss Centre for Applied Human Toxicology (SCAHT), University of Basel, Missionsstrasse 64, 4055 Basel, Switzerland
6. Molecular Nutrition and Human Physiology Laboratory, Department of Food Engineering, İzmir Institute of Technology, 35430 Urla, İzmir, Turkey
7. Kidney Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
8. Department of Organic Chemistry, Bioorganic Chemistry and Biotechnology, Silesian University of Technology, B. Krzywoustego 4, 44-100, Gliwice, Poland
9. Department of Pharmaceutical Toxicology, Yeditepe University Faculty of Pharmacy, Istanbul, Turkey
10. Department of Medicinal Chemistry, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
11. Department of Biophysics and Biochemistry, Baku State University, Baku, Azerbaijan
12. Institute of Radiation Problems, National Academy of Sciences of Azerbaijan, Baku, Azerbaijan
Received 2022-7-28; Accepted 2022-11-10; Published 2023-3-5
Abstract
Nanomaterials have been extensively studied in cancer therapy as vectors that may improve drug delivery. Such vectors not only bring numerous advantages such as stability, biocompatibility, and cellular uptake but have also been shown to overcome some cancer-related resistances. Nanocarrier can deliver the drug more precisely to the specific organ while improving its pharmacokinetics, thereby avoiding secondary adverse effects on the not target tissue. Between these nanovectors, diverse material types can be discerned, such as liposomes, dendrimers, carbon nanostructures, nanoparticles, nanowires, etc., each of which offers different opportunities for cancer therapy. In this review, a broad spectrum of nanovectors is analyzed for application in multimodal cancer therapy and diagnostics in terms of mode of action and pharmacokinetics. Advantages and inconveniences of promising nanovectors, including gold nanostructures, SPIONs, semiconducting quantum dots, various nanostructures, phospholipid-based liposomes, dendrimers, polymeric micelles, extracellular and exome vesicles are summarized. The article is concluded with a future outlook on this promising field.
Keywords: Nanoparticles, nanovectors, cancer, drug delivery, nanomaterials
Introduction
The tremendous growth in the field of nanotechnology has dramatically impacted biomedicine and drug delivery. For example, the ability to target a payload of active pharmaceutical ingredients (API) to a specific location at a proper concentration and time has driven the development of new cancer treatments (1) with the use of so-called nanocarriers or nanovectors. Such units, with size on the order of nanometers, combined with polymeric matrices, offer a more controlled release profile, which can be beneficial for the treatment (2). In particular, nanocarrier contrast agents that assist cancer diagnosis has led to breakthrough advances in cancer treatment. In this context, albumin-conjugated paclitaxel or a liposomal form of doxorubicin are among the first nano-therapeutics with great potential for cancer patients, whose performance is much improved because of nanoscopic size (3, 4).
There is plenty of motivation to develop new treatment approaches because the current methods have serious drawbacks. The use of conventional chemotherapeutic agents requires high drug doses and induces different adverse effects, which affect patients considerably, as such treatments lack precise means of targeting the cancerous cells. Nanotechnology-based drug delivery systems have opened new avenues in this context by minimizing these limitations (5). For example, nanoformulations increase the elimination half-life of the drugs and preserve them in circulation for a longer time (6). Furthermore, drugs can be better guided to cancer cells with nanomaterials (7). In addition, nanocarriers impede the degradation of medications, diminish their renal clearance, and increase the benefits of cytotoxic chemicals (1). These reasons justify why nanocarriers as intelligent drug delivery vehicles have gained significant attention for controlled drug release and bio-imaging purposes. Furthermore, nanocarriers exploiting specific receptors for targeting are particularly hopeful (5). Such nanocarriers performing as nanovectors are commonly grafted with functional groups, or amphiphilic surfactants are used to encapsulate them to boost their performance, which could be otherwise hampered by issues with solubility in the aqueous media (8, 9).
The efficiency of a nanovector-based treatment depends on cellular uptake and retention time, known as enhanced permeability and retention (EPR). Thus, the researchers focus on nanovectors with increased EPR effects to have the highest impact on cancer cells. The cellular uptake of nanovectors occurs mainly by non-transporter-guided endocytosis mechanisms. Recent studies indicate that the directed permeation of nanovectors via membrane crossing should improve the outcome of cancer treatments. Nutrient transporters are more highly expressed in cancer cells than in noncancerous cells. Thus, transporter-mediated nanovector uptake strategies are beneficial for drug delivery. Moreover, the plasma membrane surface should also be considered during the design of nanovectors since the plasma membrane is selective and semipermeable for molecules. Thus, the characteristics of nanovectors, such as size, shape, and charge, are essential. The nanoparticle size plays a significant role in cellular uptake. As a rule of thumb, the nanoparticle size should be ~ 50 nm for an efficient rate of particle internalization. Regarding charge, it has been repeatedly noticed that charged surface area of the nanoparticles enhances cellular uptake. Lastly, the eucaryotic cells have a lipid bilayer membrane structure composed of a hydrophilic head and hydrophobic tail. Because the extracellular surface of the membrane is a crucial factor in cellular uptake, when nanovectors are designed, structural features of nanovectors should be considered to ensure appropriate cellular uptake.
This review article provides an overview of recently developed nanovectors for cancer therapies to highlight the merits of this new treatment approach. Examples of such formulations are thoroughly analyzed to gauge how their characteristics impact the performance (Figure 1) and what is their utility for drug delivery. The article is concluded with a future outlook, indicating the areas of this field that require the most attention nowadays.
Overview of the scope of the article
Organic nanovectors
Liposomes
Liposomes are phospholipid-based spherical bilayer vesicles with a hydrophilic volume. They are among the most preferred nanocarriers because they can entrap hydrophilic and lipophilic drugs (10-12). A cell-like membrane shape, active group protection, low immunogenicity, safety, biocompatibility, and efficacy are among the various advantages of liposomes (13). The development of targeted liposomes by incorporating ligands such as folate, peptides, mannose, and transferrin enables the selective delivery of medications to a specific region (14, 15). As a matter of fact, the first FDA-approved nano-drug was Doxil in which doxorubicin was enclosed in a liposome.
However, liposomes elicit certain drawbacks, such as accelerated blood clearance (ABC) through the reticuloendothelial system (RES), a low entrapment rate, and a greater possibility for amphiphilic and hydrophilic drugs to escape from the liposomal vesicles, among others (16-18). In addition, their physicochemical properties affect the removal of liposomes by the mononuclear phagocyte system (MPS) and the RES.
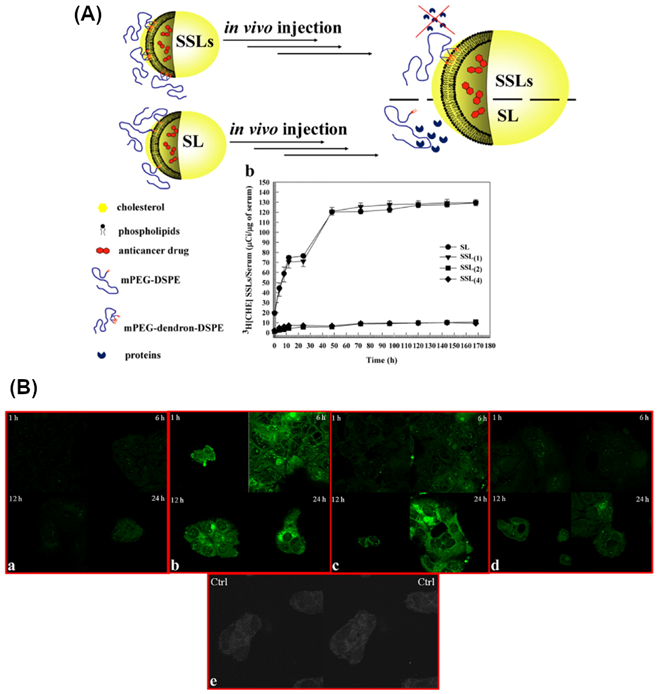
Pasut et al. (19) synthesized novel methoxy polyethylene glycol (PEG)-phospholipid structures that formed super stealth liposomes with a large anticancer agent payload (e.g., doxorubicin), a long half-life in the circulation, liposomal stability, and a good biodistribution profile. In addition, a cell culture study showed that super stealth liposomes have high intracellular uptake, acceptable toxicity, and improved therapeutic efficacy (low dose requirement of an anticancer agent) (Figure 2) (19). The promising performance was justified by using PEGylated nanoparticles which can increase drug accumulation at the desired site due to the reduction of macrophage clearance.
Moreover, the nanovectors can be made interactive. Stimulus-triggered drug delivery systems, which utilize the constituents of the tumour niche, have recently increased payloads delivered to cancerous tissue (20-23). For example, pH-stimulated liposomal formulations enhance drug release inside the tumour (24) due to different pH in the vicinity of the tumour. Paliwal et al. (25) developed hyaluronic acid-modified pH-stimulated liposomes that exhibited an increased affinity to CD44 receptor-overexpressing cells compared with normal cells.
Furthermore, liposomal systems can be used as theranostic apparatuses by simultaneously incorporating a diagnostic and therapeutic moiety (26). The second generation of liposomes containing polysaccharides, oligosaccharides, glycoproteins, and synthetic polymers on their surface exhibit even longer circulation time. To summarize, the liposomes highlighted above show enhanced blood circulation time, improved biodistribution, and greater stability and efficiency (19, 27). However, the benefits of nanovectors are not limited to spherical carriers. The merits of more complex structures will be presented below.
(A) Illustration of a super stealth liposome (SSL). The stability of the vesicle has been enhanced by attaching a single polyethylene glycol (PEG) chain to several phospholipids. When used as a drug delivery vehicle, this SSL exhibits a better biodistribution and antitumour effect than conventional stealth liposomes (SLs). The plot quantifies the stability of SSLs in serum. The leakage of 3[H]CHE from the SL and SSLs was used as a stability measurement. (B) Confocal laser scanning micrographs of CaCo-2 cells incubated with fluorescein-labelled SL and SSLs. CaCo-2 cells were treated with fluorescent SL or SSLs and incubated for different periods (1 h, 6 h, 12 h, and 24 h). a, SL. b, SSL(1). c, SSL(2). d, SSL(4). e, Control (untreated CaCo-2 cells). Modified and reproduced with permission (19). Reproduced with permission. Copyright Elsevier (2015).
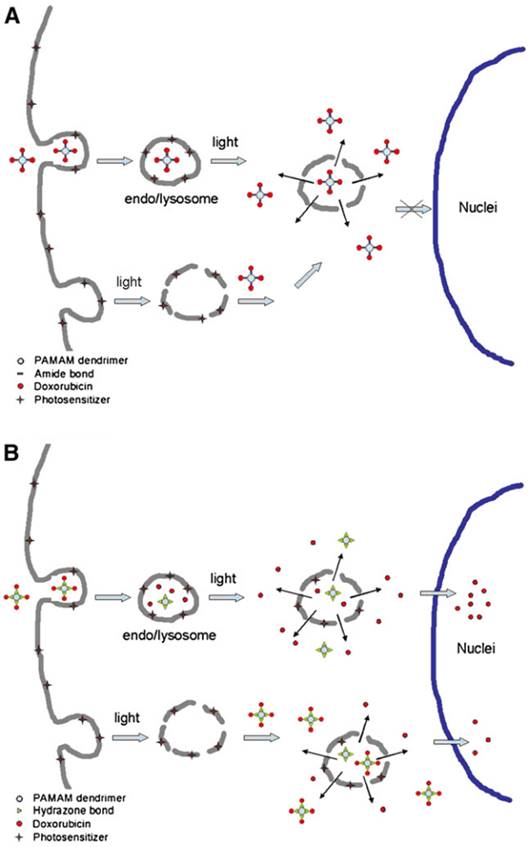
A suggested model for the cellular uptake of (A) poly(amidoamine) (PAMAM)-amide-doxorubicin and (B) PAMAM-hydrazone-doxorubicin in light-exposed Ca9-22 cells. Nuclear accumulation of doxorubicin is denoted by arrows (34). Reproduced with permission. Copyright Elsevier (2007).
Dendrimers
Dendrimers (dendrons) comprise three main parts: a repeated unit connected to the central core, the core, and functional end groups (5, 28). Different dendrimer-based nanocarriers have been utilized for cancer therapy. Currently, poly(amidoamine) (PAMAM), peptide dendrimer, polylysine, poly-l-lactide, poly-caprolactone, poly(ethylene glycol), and poly(propylene imine) are most commonly employed (29). In particular, anticancer agent-loaded dendrimers combined with iron oxide or gold nanoparticles (AuNPs) have received great attention in cancer treatment (30). Attaching targeting molecules to dendrimers can enhance the cancer-specific delivery of antitumour agents and decrease the unwanted effects. Antigens and ligands such as folate, dextran, and galactose are typically used for this purpose and are associated with the cationic cytotoxicity of dendrimers (31, 32). Light, temperature, pH, and other stimuli have been used to develop stimulus-responsive dendrimers (33).
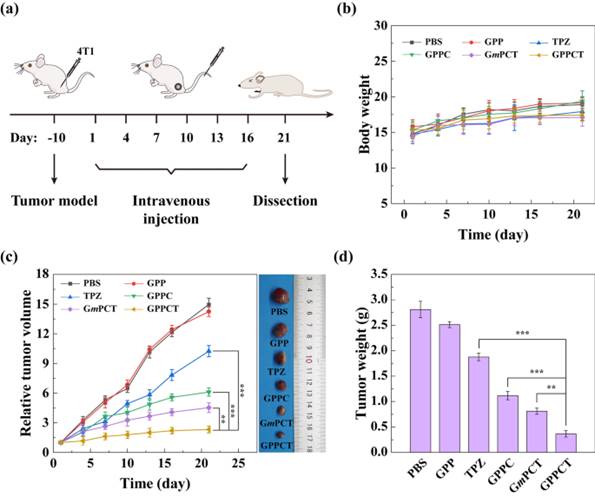
In vivo treatment of tumour-carrying mice treated with phosphate-buffered saline (PBS), G5.NHAc-PEG-PBA (GPP), G5.NHAc-PEG-PBA@Cu(II) (GPPC), G5.NHAc-PEG-PBA@Cu(II)/TPZ(CPPCT), G5.NHAc-mPEG@Cu(II)/TPZ (GmPCT), or tirapazamine (TPZ). (a) Treatment strategy. (b) Body weight. (c) Relative tumour volume. (d) Tumour weight (35). Reproduced with permission. Copyright Springer (2022).
PAMAM dendrimers conjugated to doxorubicin have been used as efficient pH-responsive nanocarriers to treat oral cancer in vitro (34). pH-insensitive PAMAM-amide-doxorubicin conjugates remain intact after liberation from vesicles and do not enter the cell nucleus (Figure 3A) (34). As shown in Figure 3B, doxorubicin is released due to the cleavage of hydrazone bonds in endosomes/lysosomes. Upon light exposure, the endosomal/lysosomal membrane is broken up, and doxorubicin translocates to the nucleus. However, earlier light exposure leads to premature breakdown of the endosomal/lysosomal membrane. Consequently, there is less cleavage of hydrazone bonds, diminishing doxorubicin release and translocation to the nucleus.
Another study evaluated the therapeutic effect of PEG-phenylboronic acid (PBA)-modified generation 5 (G5) PAMAM dendrimers in vivo in 4T1-tumour transplanted BALB/c nude mice (receiving phosphate-buffered saline [PBS], G5.NHAc-PEG-PBA [GPP], G5.NHAc-PEG-PBA@Cu(II) [GPPC], G5.NHAc-PEG-PBA@Cu(II)/TPZ [CPPCT], G5.NHAc-mPEG@Cu(II)/TPZ [GmPCT], or tirapazamine [TPZ]) (35). As depicted in Figure 4C, pure nanocarriers (GPP) could not inhibit tumour growth. Despite the tumour inhibitory effects of TPZ, its low uptake rate and fast metabolism precluded its potential therapeutic effects. On the contrary, the GPPC group acted more efficiently in a target-specific manner due to its uptake and accumulation in the tumour niche. Figure 4D shows the tumour weights for each group.
Micelles
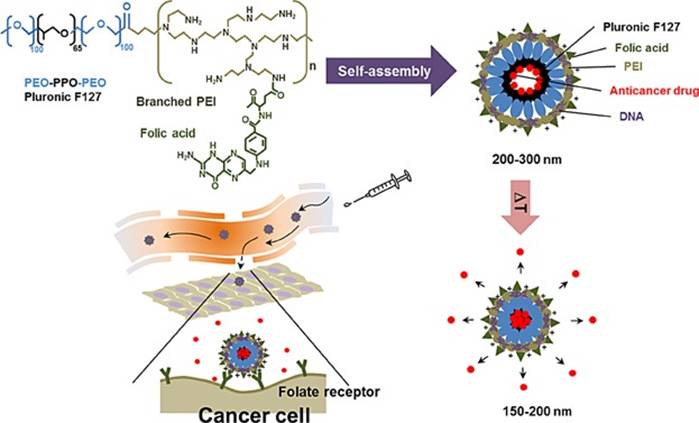
Another way to shape nanovectors is to use micelles, which can assume a broad spectrum of shapes. Polymeric micelles have recently gained significant attention and have become one of the most well-examined nanocarriers in cancer theranostics. Micelles (10-100 nm) are self-assembled structures in water with a hydrophobic core and a hydrophilic corona that can accommodate hydrophobic medications in their core (36, 37). Different ligands, including antibodies, aptamers, folate, and carbohydrates, have been implemented to decorate the micellar surface. Ultrasound, enzymatic, temperature, and pH alterations have been used to provide stimulus-responsive micellar drug delivery systems (38). The intracellular uptake of micelles can be enhanced by attaching several functional groups to the hydrophilic end of the micelles. For instance, Seo et al. (39) constructed a temperature-responsive micelle-based system for the co-delivery of genes and anticancer drugs (Figure 5), which contains crosslinked polyethyleneimine-modified micelles modified with a targeting molecule (FA). This multifunctional nanosystem exhibited no toxicity. Genes and short interfering RNAs (siRNAs) have also been efficiently delivered by polyion complex micelles (40). Amphiphilic copolymer-based micelles have been used for concomitant cisplatin and paclitaxel delivery and have notably increased loading efficiencies (41). Moreover, a papain-functionalized, mucopermeating, thiolated redox micelle has been applied for the targeted delivery of paclitaxel. This micelle-based nanoformulation inhibited the P-glycoprotein efflux pump and enhanced the bioavailability and penetration of the drug (42).
It has to be stressed that the utility of nanovectors for cancer treatment is not limited to biological molecules arranged on the nanoscale. Over the past decades, a plethora of nanomaterials has been discovered that can contribute toward solving this issue.
Nanomaterials as nanovectors
Carbon Nanotubes (CNTs)
CNTs are carbon-based cylindrical structures with an open core that might serve as valuable nanocarriers in cancer treatment (43). Single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) are classified based on the number of graphene layers utilized to construct them. Unique physicochemical properties of CNTs, such as intracellular bioavailability, an ultra-high aspect ratio, and high cargo loading (44, 45), make them applicable in this area. They are especially useful as CNTs have a longer residence time in lymph nodes than liposomes (43). Thus, they are promising nanocarriers for treating lymph node tumours (46). In this context, Yang et al. (46) developed folic acid-functionalized MWCNTs containing superparamagnetic Fe3O4 nanoparticles conjugated to cisplatin. The authors used an external magnet to guide the MWCNTs to the lymph node (46). They found that the drug was released in the tumour cells for several days (46), making the treatment approach successful.
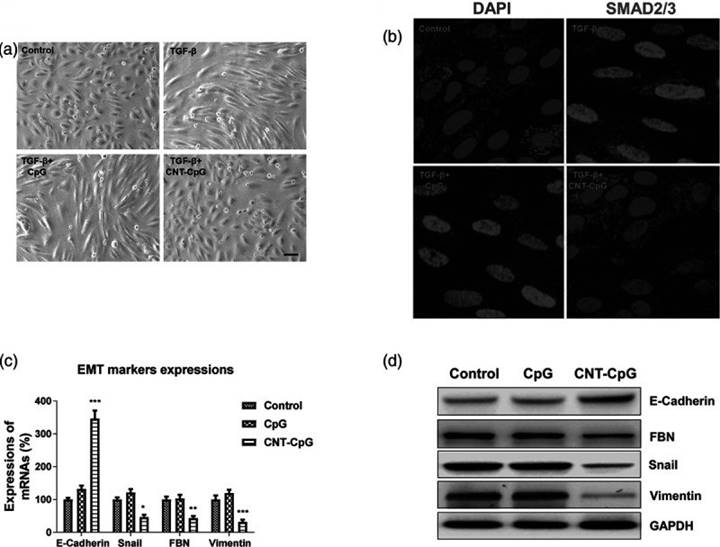
Furthermore, functionalized CNTs have been utilized as nanovectors in gene therapy to deliver biomolecules such as aptamers, microRNA (miRNA), siRNA, and DNA (45, 47). They have demonstrated high therapeutic efficacy (48, 49). Besides that, CNTs have been used in cancer immunotherapy (50). Recent experimental studies have revealed that gliomas can uptake CNT-conjugated CpG complex (CNT-CpG), which showed tumour suppressor effects in vitro and in vivo (51). Treatment of colon cancer with CNT-CpG significantly inhibited the epithelial-to-mesenchymal transition (EMT) signaling pathway and translocation of transcription factor SMAD2/3 (51). Additionally, there was a substantial decrease in the expression of EMT-related markers such as vimentin, Snail, and fibronectin (51). In another study, epithelial cell marker E-cadherin expression increased in vivo in xenograft mice tumours, while vimentin was downregulated following CNT-CpG treatment (Figure 6) (52).
The temperature-responsive micelle-based delivery system for co-delivery genes and anticancer drugs (39). Reproduced with permission. Copyright Wiley (2015).
Carbon nanotube conjugated to CpG (CNT-CpG) inhibits the transforming growth factor-beta (TGF-β)-induced epithelial-to-mesenchymal transition (EMT) of colon cancer cells. (a) Morphological alterations in HCT116 cells. (b) Treated cells were stained with DAPI and an anti-SMAD2/3 antibody. (c and d) Messenger RNA (mRNA) and protein expression of EMT marker genes were detected by real-time polymerase chain reaction and western blot, respectively (52). Reproduced with permission. Copyright Wolters Kluwer Health (2020).
Inorganic nanoparticles
Similarly to CNTs, nanoparticles with different elemental compositions, structures, and properties show encouraging potential for several biomedical applications comprising performances as nanocarriers for drug/RNA/DNA/gene delivery, as contrast agents for bio-imaging techniques, or radio-enhancing/photothermal therapeutic agents. Suitably coated gold, Al2O3, ZrO2, SiO2, MnO, Fe3O4, and Fe2O3 nanoparticles with sizes between 10 and 50 nm are not only biocompatible, non-toxic and chemically stable but also provide sufficiently large surfaces for attaching different and complementary and mutually enhancing functionalities, making them often more stable than organic materials (53).
Gold nanoparticles (AuNPs) exhibit unique features such as localized surface plasmons, adequate biocompatibility, and high affinity for thiols when used as vectors (54). Multiple synthesis strategies are used to adjust their morphological properties and surface functionalization. Turkevitch and Brust-Schiffrin (54) synthesis techniques are used to prepare gold nanospheres. Whereas Turkevich's method utilizes gold acid as a precursor and sodium citrate as a reducing agent and surfactant, Brust-Schiffrin's synthesis of thiolated AuNPs occurs via a two-phase procedure with sodium borohydride for the reduction of gold acid and tetraoctylammonium bromide as phase transfer agent. The direct use of as-introduced functional thiols and pre-coordinated thiolate ligands facilitates these nanoparticles to act as nanovector (55). Chemically modified AuNPs exhibited specific cytotoxicity to cancerous cells while sparing healthy cells. In this context, the dose, the nature of the ligand, the sizes of the AuNPs, and the administration route determine the cytotoxicity, which should be taken into account while designing AuNP-based nanovectors for cancer treatment (54).
Due to their competitive performance, AuNPs have attracted increasing attention in developing different nanocarriers for drug delivery, tumour sensing, and photothermal cancer therapy (56). They exhibit several unique features that make them promising theranostic cancer agents. The inactivity of AuNPs towards biological systems is among their most prominent advantages compared with metal-oxide-based nanoparticles (57). Besides that, AuNPs perform excellently as plasmonic heating agents by converting near-infrared (NIR) light into heat and are thus best suited for application in photothermal therapy of malignancies. AuNPs with proper sizes and shapes exhibit no phototoxicity, so they may be considered biocompatible agents (58, 59).
Concomitantly, the implementation of high atomic number (Z) materials to increase sensitivity to X-ray irradiation has been studied for over half a century. High-Z materials do emit not only secondary X-rays but also Auger, Compton, and photo-electrons which induce DNA damage and ionize water, producing reactive oxygen species (ROS). These phenomena may be used for cancer treatment (60). In vitro and in vivo studies confirmed that AuNP-based radiation therapy eradicates tumuor cells with high efficiency (61, 62).
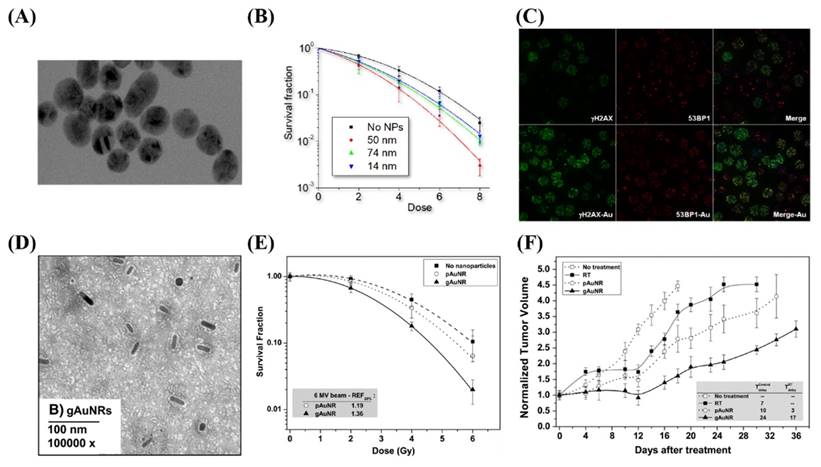
Enhancing the X-ray irradiation dose by AuNPs was first established in an in vivo experiment using 1.9 nm AuNPs (63). In this study, 30 Gy X-ray irradiation significantly reduced the volume of mice's subcutaneous tumours. However, Hainfeld et al. utilized high concentrations of AuNPs (2.7 g/kg), a dose that is not clinically feasible. AuNPs with sizes smaller than 2 nm were observed to invade deep-lying tumours, which boosted the local X-radiation dose to maximum therapeutical efficacy. In the case of successful internalization of AuNPs, radiosensitization is feasible even at low concentrations (64, 65). Chithrani et al. (66) reported increased radiosensitization at 6 MV by means of 50-nm spherical AuNPs (Figure 7).
Furthermore, AuNP-conjugated methotrexate exhibits higher cytotoxicity towards tumour cell lines compared with free methotrexate. Doxorubicin bound to AuNPs by acid-labile bonds shows increased toxicity to breast cancer cells line (68-70). It was also reported that the conjugation of AuNPs with fluorescent heparin as a targeting agent creates a promising material cancer diagnosis (71, 72). One should also keep in mind that AuNPs provide enhanced superoxide radical anion generation and therefore induce cell death directly (72).
To make this approach more sustainable, herbal or biogenic strategies have also recently been applied to produce AuNPs. For example, AuNPs were prepared from Abies spectabilis and as-produced material exerted an anticancer effect on bladder cancer (73). AuNPs are cytotoxic for bladder cancer cell lines (T24) by stimulating apoptosis, nuclear DNA fragmentation, and DNA injury (74). Upon NIR or visible light irradiation, AuNP exhibit localized surface plasmon resonance (LSPR) that is highly sensitive to environmental changes, a phenomenon that enables applications in imaging techniques for cancer diagnosis.
The use of gold nanoparticles (AuNPs) to enhance the lethal impact of X-rays on tumour tissue. (A) A micrograph of spherical AuNPs. (B) The clonogenic assay showed that the largest effect occurred with 50-nm AuNPs. (C) Confocal micrograph showing the uptake of AuNPs. (D-F) Increased radiosensitization of Graphene nanoribbons (GNRs) in vitro in prostate cancer cell lines and in vivo by measuring the tumour volume. Reproduced with permission from (67). Copyright Elsevier, 2015.
Construction of a multi-component cancer treatment agent based on MSNs depicted in the top left corner. (B) The mechanism of action in vivo. Reproduced with permission from (91). Copyright American Chemical Society (2016).
In addition, suitably functionalized AuNPs allow the detection of abnormal sequences of amino acids via hybridising techniques. Antibody-conjugated AuNPs facilitate tumour-targeting and thereupon accumulation of the AuNPs in cancerous tissue. AuNPs when acting as drug nanocarriers can perform either in active or passive way (75). In active vectorization, AuNPs bind to overexpressed receptors on the plasma membrane of cancer cells and are subsequently endocytosed. In passive vectorization, drugs are conjugated to AuNPs. In summary, AuNPs are among the most prevalent type of nanoparticles to modulate cancer because they act as multifunctional and safe materials in cancer theranostics (76).
Silver nanoparticles (AgNPs) have also shown great potential due to their antimicrobial, antiviral, and anticancer nature. Due to these properties, AgNPs are very widely used. It has been reported that AgNPs induce cytotoxicity via apoptosis and necrosis in different cancerogenic cells and exhibit results against secondary effects of current therapies (e.g. deoxyribonucleic acid (DNA) damage, generation of reactive oxygen species, increasing leakage of lactate dehydrogenase). In addition, AgNPs have shown some unique properties, such as ten times greater light scattering cross-section than AuNPs (77), making these particles very interesting for their biosensors (78, 79). AgNPs can also show great potential as photo-controlled drug delivery vectors due to their more significant extinction coefficient and blue-shifted plasmon resonant peak (80).
Besides nanostructures from noble elements, simple metal oxide NPs (MONPs) also exhibit favorable characteristics since they are chemically stable and easy to process to the desired size, shape, and porosity. In addition, they may be incorporated into hydrophobic and hydrophilic systems and can be facilely functionalized with various molecules via covalent or electrostatic binding (81). MONPs can be classified based on the dimensionality into 0- (e.g. nanocluster, quantum dots), 1- (e.g. nanorods, nanowires), 2- (e.g. nanoshets), and 3-dimensional (e.g. scaffolds) materials (82), which further increases their application potential as changing the shape dramatically modifies the biological properties. What is important, many MONPs, for example, TiO2, ZnO, ferric oxide (Fe2O3), and ferrous oxide (Fe3O4), appear to be safe for mammals (82). They perform well as nanocarriers with excellent delivery efficacy, as they can invade the body via respiration, ingestion, skin infusion, or even direct injection (81).
Mesoporous Silica Nanoparticles (MSNs)
Another vital factor affecting biological performance is the material's porosity. Mesoporous silica nanoparticles (MSNs) possess an exceptional potential as versatile nanoplatforms for cancer theranostics (83-86) because of high biocompatibility, uniform pore size distribution, large pore volume, substantial surface area, and the capability for further chemical modifications. The mesoporous surface of the silica nanoparticles provides exceptional opportunities for drug loading and its stable release (87, 88). Mobil Composition of Matter 41 (MCM-41) is the most studied MSN for cancer treatment, which simultaneously is the most promising silica-based material for this application (89). It was found that the performance of MSNs can be extended by coating them with PEG, thus making long-circulating nanocarriers (90).
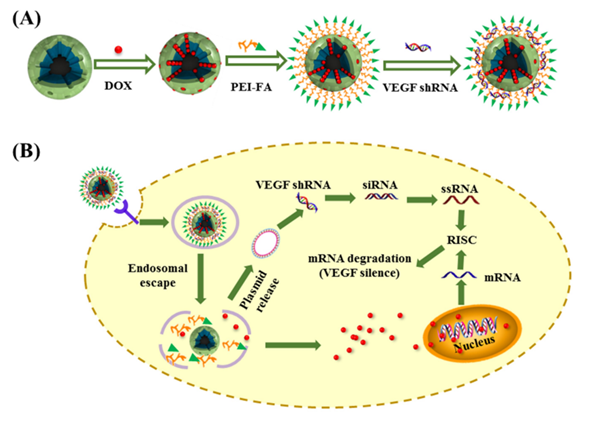
Folate-modified MSNs have shown significant cytotoxic effects against human breast and cervical cancer cells (91, 92). Li and colleagues reported the construction of MSN-based nanocomplexes composed of MSNs, doxorubicin (DOX), polyethyleneimine (PEI)-conjugated folic acid, and Vascular Endothelial Growth Factor shRNA that enabled co-delivery of chemotherapeutics and nucleic acid drugs to improve cancer treatment (Figure 8). MSNs are also suitable carriers for nucleic acid-guided treatments and nucleic acid delivery to tumour cells (93-97). They have recently been applied in photodynamic and photothermal therapy of different cancer types, reaching appreciable performance (97).
Superparamagnetic Iron Oxide Nanoparticles (SPIONs)
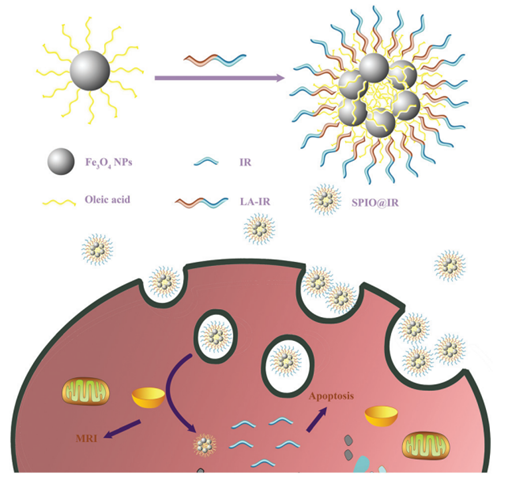
An important aspect of any treatment is the ability to direct it to the right location. One of the types of materials that can be guided and accumulated in tumour tissue by applying an external magnetic field are superparamagnetic iron oxide nanoparticles (SPIONs), which have recently received considerable attention from the scientific community (98). Suitably functionalized SPIONs are biocompatible, and they have high application potential due to their properties. For example, they are excellent contrast agents for magnetic resonance imaging (MRI) and can acs as magnetic hyperthermia agents under an altering magnetic field (99). The scheme below displays how SPIONs can be used for nanotheranostics in cells (Figure 9).
Generation of bio-compatible SPIONs and their interaction with cells. Abbreviations: Fe3O4 NPs, superparamagnetic iron oxide nanoparticles; IR, irinotecan; LA-IR, amphiphilic lauric acid-irinotecan prodrug; SPIO@IR, LA-IR inserted SPIO prodrug. Reproduced with permission from (98). Copyright Royal Society of Chemistry (2018).
Because bare SPIONs agglomerate in aqueous solutions, they must be stabilized to form stable aqueous colloids (100). Solar and co-workers (101) reported coating SPIONs with poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) as a biodegradable and biocompatible polymer to avoid toxicity while preserving all of its potential applications. Recent studies in the realm of drug delivery and tissue engineering have confirmed that PHBV (101) is a cost-effective material that shares similar physicochemical properties with the majority of widely used polymers (102-104).
The size of a SPION-based drug delivery system should be 10-100 nm for in vivo application to avoid renal clearance and RES (105). To extend their applicability, SPIONs can be conjugated with different kinds of biocompatible materials such as liposomes, polymers, and viral vectors. Non-viral drug delivery via surface-modified SPIONs has recently undertaken outstanding developments (106, 107), and they can specifically reach tumour sites (108). Moreover, the use of targeting moieties conjugated to SPIONs has resulted in a significant reduction in the required and off-target effects (109).
Quantum Dots (QDs)
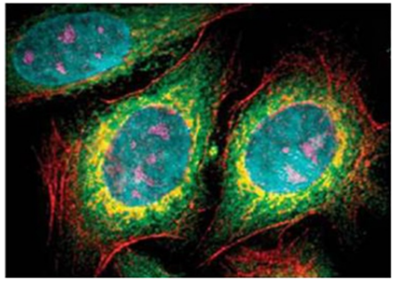
There are other shapes of nanoparticles that can also be helpful in cancer theranostics. QDs, including Si QDs, ZnO QDs, and ZnS QDs, possess interesting optical, electrical, and fluorescence properties (110). Fig. 10 shows how multi-color imaging of fixed human epithelial cells can be accomplished using five different color QDs.
The multi-color imaging of fixed human epithelial cells using five types of QDs. Adapted with permission (110). Copyright Springer (2015).
With appropriately modified surfaces, water-soluble ultra-small QDs (2-4 nm) can be produced (111) and employed in nanocarriers to visualize tumour tissue and concomitantly release a drug in the desired region. Commercially available QDs comprise three components: a core (a semiconductor material), a shell, and functional ligands. The core and surrounding shell are semiconductor materials, and caps enclose double-layered QDs (112). Functionalising QDs with biocompatible polymeric materials or targeting moieties can substantially improve their performance (113, 114). Furthermore, QDs escape from the RES and renal clearance due to their small size, so their blood circulation time increases (80).
Senel and colleagues (115) developed N-doped graphene quantum dots as cost-effective materials that show antioxidant and anti-inflammatory effects. According to their results, N-doped graphene QDs could be linked to DNA via electrostatic interactions or intercalation. The graphene QDs easily penetrate cancer cells and eradicate them with high efficiency. siRNA can be integrated into the QD structure to produce agents that are effective in cancer treatment even at low doses (115). Akbarzade et al. (116) synthesized an aptamer-functionalized mesoporous silica-coated QD. The nanohybrids are suited for MRI and fluorescent imaging. In another study, researchers utilized GQDs for drug delivery in cancer treatment (117). Javanbakhta et al. (118) obtained nano-carboxymethyl cellulose (CMC)/GQD hydrogels with superb mechanical and rheological properties. CMC/GQDs showed high permeability, negligible toxicity, and exerted a prolonged doxorubicin release profile. Antibody-linked graphene QDs nanoparticles have been used to deliver cisplatin and targeted cellular imaging (119). In addition, Fe3O4@SiO2@GQD-FA nanoparticles have synergistic effects with doxorubicin in Hela cells (120). Overall, the large volume of research on QDs for cancer theranostics validates their substantial application potential for this purpose.
Nanoshells, Nanowires, and Cantilevers
Other more complex shapes of nanoparticles also have their utility for cancer theranostics (121). For instance, nanoshells exhibit increased permeation and retention effects due to their small size (Figure 11), so they accumulate preferentially at the tumour site (121). Results from in vivo studies have demonstrated that nanoshell administration could be considered a low-toxic approach (122). Conjugation of nanoshells with biomolecules facilitates the recognition of the tumour location and spares healthy cells. The ability of nanoshells to absorb different energy sources, such as light and radio frequency, has turned them into potent tumour cell killers (123).
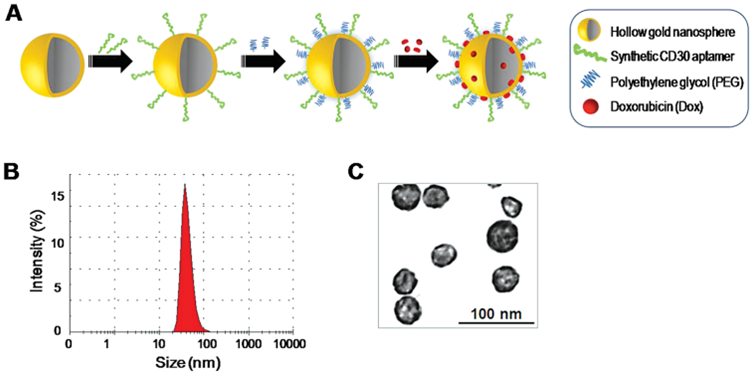
Furthermore, some nanoshells absorb NIR light, and hence they can act as a plasmonic heater to produce the heat required for photothermal therapy of specific tumour cells. Liang et al. (123) synthesized doxorubicin-functionalized gold nanoshells that release the drug into the tumour tissue in a pH-dependent manner. Gold nanoshells can also be conjugated to different targeting moieties that enhance cellular uptake of the drug. In this context, aptamer-functionalized doxorubicin-loaded gold nanoshells have been shown to bind specifically to CD30-overexpressed lymphoma cells. The acidic pH of lysosomes triggers drug release from this system (124).
Simultaneously, microfluidic channels can be used to house nanowires for sensing molecular signatures of analytes that flow through the channel and relay this information to the external electrodes. Nanowires are highly specific and selective sensing devices that can detect the presence of altered genes in a distinct cancer cell type (125). Peng et al. (126) reported silicon nanowires developed to deliver docetaxel as an ultra-performing nanovector. This nanocarrier had an ultra-high drug-loading capacity and exhibited a substantial anticancer effect both in vitro and in vivo. Kuei-Chang et al. (127) developed the first PEGylated copper nanowire for photothermal treatment. It converts NIR light to heat with good flexibility and reproducibility. In addition to the effect of this nanowire in vitro, its intra-tumoural injection significantly impaired colon cancer growth.
Lastly, cantilevers are lithographic structures that generate flexible microscopic beams and can be coated with different molecules to provide a platform for detecting distinct disease markers, including cancer (128). In other words, the tip mounted at one end of a cantilever can be attached to diagnostic molecules that, in turn, may detect specific DNA-binding proteins expressed by certain cancer types. Wu et al. (128) developed a nanocantilever that can detect PSA at levels lower than the clinically relevant threshold value. This method is cost-effective, highly sensitive, and potentially more effective than enzyme-linked immunosorbent assay (ELISA).
Extracellular vesicles (EVs)
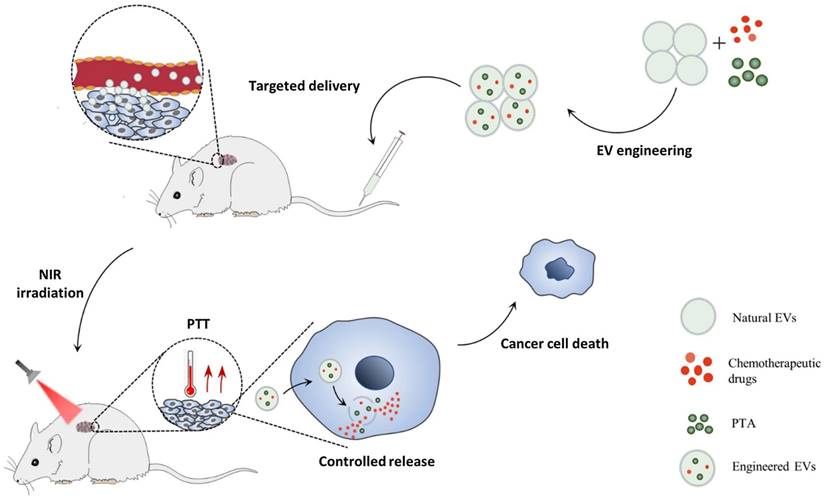
EVs are a heterogeneous group of lipid bilayer membrane-enclosed vesicles that provide cell-cell communication via the transmission of different cargo. Small extracellular vesicles (sEVs) have gained importance as innovative, safe, natural, and efficient delivery systems in the treatment of diverse diseases, in particular cancer. Figure 12 depicts a recently engineered EV vehicle for the co-delivery of photothermal agents (PTAs) and drugs. Different types of sEVs are discussed in the following subsections.
Cancer Cell-Derived sEVs
Cancer cell-derived sEVs have been considered appealing delivery vehicles of different anticancer agents because they accumulate in tumours due to the cellular tropism of sEVs (130, 131). Thus, autologous sEVs are mainly used and will be discussed here. Cancer cell-derived sEVs can transfer tumour antigens to dendritic cells, stimulating the immune response against cancerous cells (132). Using special sEVs as drug carriers substantially enhance the efficacy of the pharmaceutical in different cancer types (133, 134). For example, the incubation of prostate cancer cell-derived EVs with paclitaxel significantly elevated the cytotoxic effect of paclitaxel in autologous parental prostate cancer cells after endocytosis (133). In another study, gemcitabine-loaded pancreatic cancer cell-derived sEVs exhibited a more significant accumulation of drugs after intravenous injection to pancreatic cancer cell xenograft mice (134). Emam et al. (135) investigated the cellular uptake of sEVs derived from colorectal C26- and melanoma B16BL6 cells by each cell type. They showed that the uptake of sEVs is larger in donor cells than in recipient cells. Kim et al. (136) confirmed the cellular tropism of sEVs. They evaluated the cellular uptake of ovarian cancer cell line (SKOV3)-derived sEVs by SKOV3 and epithelial cell (HEK293). These sEVs accumulated more in the SKOV3 cell line to a larger extent (136). Although using autologous sEVs has been considered a safe technique, one should be aware of the immune-inhibitory effects of cancer cell-derived sEVs (134). The origin of sEVs and their cargo determine their inhibitory or stimulatory effects on the immune system. Moreover, cancer cell-derived sEVs might also increase tumour cell growth and metastasis and induce drug resistance (133). Therefore, additional studies are required to evaluate the possible tumour stimulatory effects.
(A) formation of gold nanosphere-based agent, (B) determination of size by dynamic light scattering, (C) TEM micrographs of the obtained material. Adapted with permission (124). Copyright Wiley (2013).
Chemo/photothermal therapy by engineered extracellular vesicles (EVs) that co-deliver drugs and photothermal agents (PTAs) to tumour sites (129). Reproduced with permission. Copyright Springer (2022).
Mesenchymal stem cell (MSC)-derived sEVs
MSCs are multipotent stem cells with extensive use in regenerative medicine due to their exceptional properties, such as self-renewal and capacity to differentiate into targeted lineages (137, 138). MSC-derived sEVs have attracted great attention for their therapeutic potential in cancer treatment, in addition to their use in regenerative medicine (138). These sEVs can also carry pharmaceutical agents. The expression of distinct receptors for humoral factors, including cytokines, growth factors, and chemokines in the tumour niche, enables them to develop tumor-suppressing effects (139). Moreover, the presence of inflammatory factors in the tumour microenvironment can stimulate the migration of MSCs into cancerous regions (140). MSC-derived sEVs exhibit paracrine effects by transmitting their properties to recipient cells. Therefore, they can serve as efficient nano-sized vectors for treating cancer (141). Bone marrow, placenta, adipose tissue, and amniotic fluid can be the source of sEVs (142). Previous reports have shown the therapeutic capacity of sEVs in treating therapy-refractory graft-versus-host disease (143). Moreover, their effectiveness in treating acute ischaemic stroke is being evaluated in an ongoing clinical trial (NCT03384433). MSC-derived sEVs containing paclitaxel have also been shown to suppress tumour growth (144).
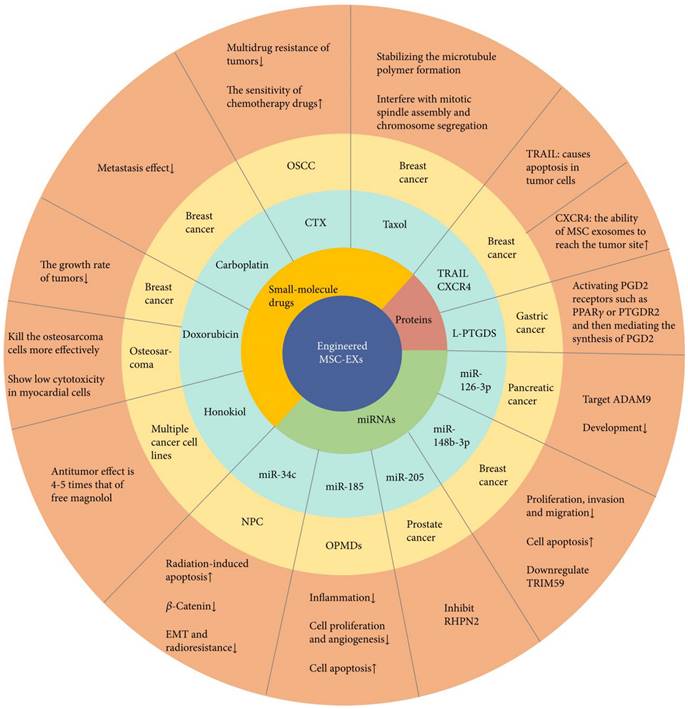
Short RNA sequences, small-molecule drugs, and proteins are the main types of cargo delivered by engineered exosomes (145). However, few studies have addressed the successful loading of proteins, which might be due to their high molecular weight, the uncertain mechanism of protein sorting by exosomes, and the absence of effective loading techniques (146, 147). The first report on protein loading into EVs for cancer treatment was by Mizrak et al. (148) in 2013. They prepared microvesicles carrying a suicide mRNA/protein that hampers the growth of a schwannoma tumour. Later, they developed a method for protein loading based on the evolutionarily conserved late-domain (L-domain) cascade. Using this technique, they loaded WW tag-labeled Cre recombinase into exosomes (146). In another study, researchers transfected MSCs with tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) and chemokine receptor type 4 (CXCR4) via exosomes. They showed synergistic effects with carboplatin in vivo (149). Exosomes have been utilized to deliver lipocalin-type prostaglandin D synthase (L-PTGDS) to gastric cancer cells (150). L-PTGDS overexpression has been associated with reduced gastric cancer growth, reduced formation of tumour clones, and reduced migration of cancer cells, and thus can act as a possible therapeutic agent. L-PTGDS shows its potential therapeutic effects by mediating the production of prostaglandin D2 and stimulating PGD2 receptors, including Peroxisome proliferator-activated receptor-gamma on the surface of tumour cells. Figure 13 illustrates the role of exosomes for the delivery of RNA, drugs, and protein according to previous research activities (151).
Although a few clinical studies have been devoted to anticancer effects of MSC-derived sEVs, these effects are still debated because MSCs per se can increase tumour cell proliferation and induce angiogenesis (138). For example, MSCs can boost breast cancer metastasis (152). Therefore, the use of sEVs harvested from MSCs should be carefully considered. So far, researchers have noted that the cell cultivation platform, tumour model, and tumour microenvironment might determine the protumour or antitumour effects of MSC-derived sEVs (153).
Immune Cell-Derived sEVs
Immune cells such as macrophages, T cells, dendritic cells, and natural killer (NK) cells also provide sEVs that could be used for drug delivery (154). These cells can naturally regulate several antitumour immune responses, enhancing therapeutic efficiency. Dendritic cell-derived sEVS are promising candidates for nanovectors for cancer therapy, as the approval of several clinical trials has confirmed their efficacy, in particular, for antitumour vaccination (155, 156). Nevertheless, it should be noted that these sEVs might lead to immune inhibition and thus cancer metastasis (157).
Engineered mesenchymal stem cell exosomes (MSC-EXs) for cancer treatment. These structures carry microRNAs (miRNAs), small-molecule drugs, and proteins (151). Reproduced with permission. Copyright Hindawi (2021).
Typical properties and anticancer implementation of natural killer (NK) cell-derived extracellular vesicles (EVs). NK EVs bind cancer cells via NKG2D-MICA/B and promote cytotoxicity by releasing their cytotoxic protein cargo. In addition, engineered NK EV-coated nanoparticles have been used to deliver anticancer agents (164). Reproduced with permission. Copyright Frontiers (2021).
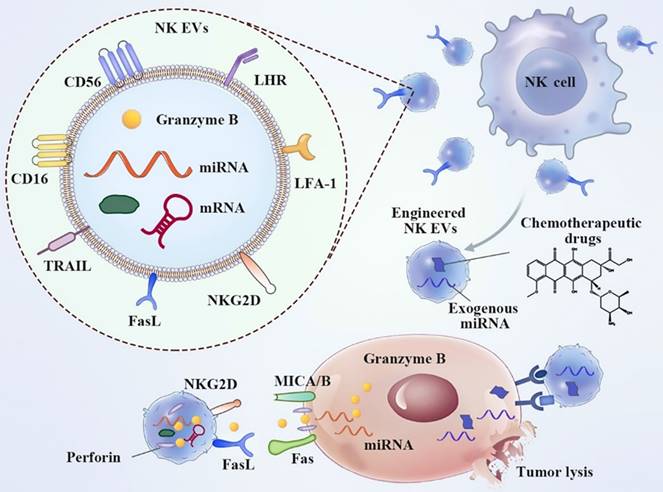
NK cells are innate immune effectors that play a pivotal role in organ immunosurveillance, microorganism-related infections, and cancer (158). Human leukocyte antigen (HLA)-linked inhibitory receptors expressed on membranes stringently control the activity of NK cells under steady-state conditions (159). NK-derived EVs encompass NK cell surface receptors (Figure 14). EVs released from active NK cells can induce apoptosis in cancer cells. On the contrary, NK ligand-bearing cancer cells decrease the expression of active receptors, including NKG2D, and block NK cell degranulation, leading to compromised toxicity and diminished levels of lytic proteins (160). Many cytotoxic proteins exist, including granzyme, perforin, FasL/CD178, small antimicrobial peptides, granulysin, and TRAIL (161). Direct killing pathways are then activated and target different types of cancer (162, 163).
As mentioned above, the majority of surface receptors are expressed on immune cell-derived EVs and their parental cells, which allow them to detect target cells and liberate their cargo. For example, proteins involved in the co-stimulation of T cells (CD80, CD86, and Intercellular Adhesion Molecule 1) can also be accumulated in EVs derived from dendritic cells (165), or EVs released from macrophages can transfer their surface antigens to dendritic cells and thus stimulate CD4+ T cell activation (166). Moreover, it is possible to modify the surface of EVs via engineering methods, especially genetic engineering. Tian et al. (167) modified the surface of dendritic cell-derived EVs by introducing iRGD peptide-bound pEGFP-C1-RVG-Lamp2b plasmid to deliver doxorubicin to breast cancer cells (168). Chimeric antigen receptor (CAR)-carrying EVs encoding mesothelin-targeted and Myc-tagged scFv have successfully inhibited the growth of MSLN-positive triple-negative breast cancer cells (167). Genetic engineering techniques transfer molecules such as Interleukin 4, indoleamine 2,3-dioxygenase, and FasL into dendritic cells, targeting distinct tumours (164, 169). Modulation of the phospholipid membrane composition or the addition of a targeting antibody on the surface of EVs is another feasible strategy. Chemical crosslinking can be used for membrane engineering. Therefore, immune cell-derived EVs in the peripheral blood might be disease-specific biomarkers of tumourigenesis and inflammation. In addition, immune cell-derived EVs combined with chemotherapeutic agents can prohibit tumour growth.
Plant exosome-like nanovesicles
Lastly, innately bioactive molecules in plant exosome-like nanovesicles (PELNVs) are suitable, efficient nanocarriers for medical applications. The role of PELNVs in regulating immunological reactions for hemostasis of the gastrointestinal system has been widely studied (170). In cancer therapy, PELNV-derived re-engineered nanovectors target cancer cells as non-immunogenic vehicles (171). For example, ginger-derived ELNVs were successfully used to deliver doxorubicin to colon tumour cells. Interestingly, the antitumour effect was attributed to doxorubicin as well as the inherent nature of this plant-based nanovector that suppresses oxidative stress and the release of inflammatory cytokines. In addition, the researchers proposed that ginger-derived nanovectors increase the release of doxorubicin in the acidic niche of the tumour and thereby diminish its systemic side effects.
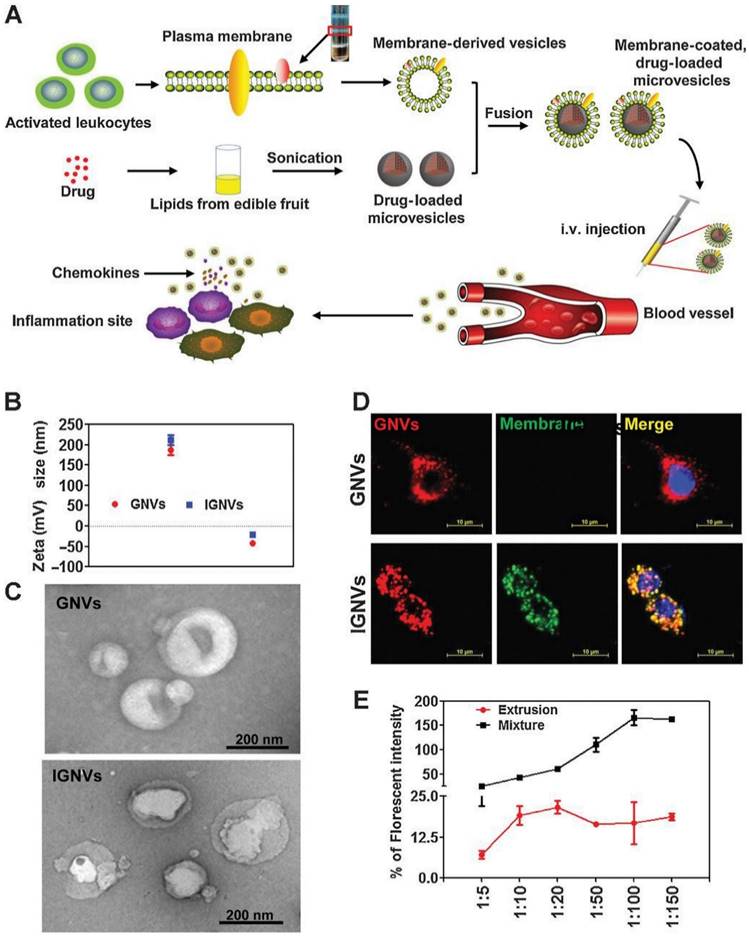
Moreover, studies showed distinct advantages of these nanovectors, such as being biocompatible, cost-effective, and innocuous (172). Grapefruit-derived nanovectors have also been developed for effective and simultaneous delivery of paclitaxel and folate to the SW620 and CT26 colon cancer models in immunodeficient mice. Of note, this nanovector did not traverse the placental barrier in intravenous injection in vivo (173). Wang et al. (174) developed a chemokine receptor-enriched plasma membrane-coated grapefruit-derived nanovector, which could efficiently deliver curcumin (an anti-inflammatory agent) and doxorubicin to inflamed CT26 colon tumours and 4T1 breast tumours in mouse models (Figure 15).
The plant source of PELNVs determines the therapeutic effects of these nanovectors. For example, hydrolysis of naringin (the key flavanone of grapefruit) to naringenin by gut microbiota facilitates the antitumour effects of grapefruit-derived nanovectors (175). Furthermore, PELNVs can inhibit the signaling cascades that lead to the expression of proteins involved in tumour development. In this context, PELNVs can disrupt the expression of cyclin D1 mRNA by activating cyclic guanosine monophosphate (cGMP)-dependent protein kinase and thus exert antitumour effects (176). PELNVs also can increase the expression of pro-apoptotic factors and suppress angiogenic molecules (177). Thus, bearing in mind all of these aspects, a combination of natural PELNVs and chemotherapy could serve as a viable plan to eradicate cancer in the future.
Currently, three clinical trials investigate the effects of PELNVs: grape-derived ELNVs for the prevention of oral mucositis in combination with chemoradiation therapy for head and neck cancer (NCT01668849), aloe- and ginger-derived ELNVs for the treatment of polycystic ovary syndrome (PCOS) (NCT03493984), and curcumin-containing PELNVs in the treatment of colon cancer (NCT01294072).
Influence of size, shape, and surface character of nanovectors for drug delivery applications
Size, shape, and surface chemistry of nanovectors are the key determinants of their drug delivery capacity (178, 179). To achieve a long circulation time, the optimum size range of nanovectors is between 20-200 nm. Nanoparticles smaller than 20 nm are rapidly cleared by the kidney, while nanoparticles larger than 200 nm are easily uptaken by macrophages (180-184). Particles larger than 3 µm in diameter can obstruct microvascular capillaries and also exhibit high macrophagic uptake (184-186).
Moreover, the shape can also influence the delivery properties of nanovectors. Recent studies on particles with different shapes have shown an evident influence of the shape on circulation, lifetime, and macrophage clearance (187). While spherical shapes tend to follow hydrodynamic forces with little lateral movement, non-spherical particles such as rods can undergo some lateral drift (188, 189).
In addition, the nanovector surface structures decide over protein corona formation and cellular uptake mechanism. For example, surface charges or short dense steric surface stabilizers (e.g. poly(ethylene glycol) (PEG)) were reported to increase the mucosal layer and, thereupon, the blood-brain barrier penetration (190, 191).
Conclusions and Future Outlook
In this review article, a wide variety of auspicious nanovectors for application in multimodal cancer therapy and diagnostics are discussed in terms of mode of action and pharmacokinetics. Promising nanovectors are made of diverse formulations including gold nanostructures, SPIONs, semiconductor quantum dots, mesoporous silica nanoparticles, phospholipid-based liposomes, PAMAM dendrimers, polymeric micelles, extracellular and exome vesicles. Surface functionalization and drug loading of these nanovectors are achieved by conjugation with anticancer drugs, targeted molecules, DNA, siRNA, aptamer, and other functional biomolecules. Depending on the nanovector material and surface chemistry, the drug release inside tumour tissue can be triggered by ultrasound, NIR light pulses, X-rays, or pH value changes. Applications of organic or inorganic nanovectors in cancer therapy include chemotherapy, radiotherapy, immunotherapy, magnetic hyperthermia, and photodynamic therapy, while nanovectors containing gold or SPIONs iron oxide can be in addition used as CT and MRI contrast agents to visualize tumor tissue and metastases.
Characterization of plasma membrane-coated grapefruit-derived nanovectors (IGNVs) characterization. (A) Preparation and drug loading steps. (B) Surface zeta potential measurement. (C) Scanning electron microscopy free GNVs (top) and IGNVs (bottom). (D) Co-localisation of the EL4 cell-derived plasma membranes and GNV cores. (E) Fluorescence resonance energy transfer-based measurements of IGNV formation. Reproduced with permission. Copyright Elsevier (2019).
For future applications in cancer therapy, nanovectors should comprise at least 2 functionalities, attack tumor cells at 2 to 3 targets, and enable targeted cancer therapies. For example, iron oxide and gold nanostructure-based nanovectors should possess surface structures enabling not only binding to anticancer drugs but also conjugation to tumor-cell targeting aptamers, metabolites, or biomolecules to make such treatment most effective. These multifunctional nanovectors may serve first as MRI or CT contrast agents and subsequently be used for tumor tissue visualization. Alternatively, they can be applied for local enhancement of radiotherapy, chemotherapy, hyperthermia, and photodynamic therapy. In the case of nanovectors based on micelles, dendrimers, or vesicles, which are also equipped with tumor-targeting functional groups but encapsulate anticancer drugs, the controlled release of the drugs inside tumor cells plays a particularly important role. Depending on the location of the tumor in the body, the treatment can be triggered with sound waves or NIR laser radiation. Moreover, hyperthermia or photodynamic therapy can be driven by a temperature increase to guarantee a controlled release of the drug payload.
In light of the preceding, there is a myriad of nanomaterials that can redefine the field of cancer treatment in the upcoming future. Given their unique properties, their application as nanovectors seems highly viable, judging by the outcomes of the initial studies described above. The realization of this vision currently hinges upon sufficient demonstration of the lack of toxicity and the development of scalable methods of their synthesis to deploy this concept in cancer theranostics.
Acknowledgements
D.J. would like to thank the National Science Centre, Poland (under the SONATA program, Grant agreement UMO-2020/39/D/ST5/00285).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ashrafizadeh M, Saebfar H, Gholami MH, Hushmandi K, Zabolian A, Bikarannejad P, Hashemi M, Daneshi S, Mirzaei S, Sharifi E, Kumar AP, Khan H, Hossein HHS, Vosough M, Rabiee N, Thakur VK, Makvandi P, Mishra YK, Tay FR, Wang Y, Zarrabi A, Orive G, Mostafavi E, Doxorubicin-loaded graphene oxide nanocomposites in cancer medicine: stimuli-responsive carriers, co-delivery and suppressing resistance, Expert Opinion on Drug Delivery 2020 19:4, 355-382
2. Huda S, Alam MA, Sharma PK. Smart nanocarriers-based drug delivery for cancer therapy: An innovative and developing strategy. Journal of Drug Delivery Science and Technology. 2020;60:102018
3. Yang J, Lv Q, Wei W, Yang Z, Dong J, Zhang R. et al. Bioresponsive albumin-conjugated paclitaxel prodrugs for cancer therapy. Drug Delivery. 2018;25(1):807-14
4. Yao S, Janku F, Koenig K, Tsimberidou AM, Piha-Paul SA, Shi N. et al. Phase 1 trial of ADI-PEG 20 and liposomal doxorubicin in patients with metastatic solid tumors. Cancer medicine. 2022;11(2):340-7
5. Hossen S, Hossain MK, Basher M, Mia M, Rahman M, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. Journal of advanced research. 2019;15:1-18
6. Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. Journal of controlled release. 2011;153(3):198
7. Tang L, He S, Yin Y, Liu H, Hu J, Cheng J. et al. Combination of Nanomaterials in Cell-Based Drug Delivery Systems for Cancer Treatment. Pharmaceutics. 2021;13(11):1888
8. Cai H, Dai X, Wang X, Tan P, Gu L, Luo Q. et al. A nanostrategy for efficient imaging-guided antitumor therapy through a stimuli-responsive branched polymeric prodrug. Advanced Science. 2020;7(6):1903243
9. Xu K, Wang M, Tang W, Ding Y, Hu A. Flash nanoprecipitation with Gd (III)-based metallosurfactants to fabricate polylactic acid nanoparticles as highly efficient contrast agents for magnetic resonance imaging. Chemistry-An Asian Journal. 2020;15(16):2475-9
10. Jain A, Kumari R, Tiwari A, Verma A, Tripathi A, Shrivastava A. et al. Nanocarrier based advances in drug delivery to tumor: an overview. Current drug targets. 2018;19(13):1498-518
11. Alwattar JK, Mneimneh AT, Abla KK, Mehanna MM, Allam AN. Smart stimuli-responsive liposomal nanohybrid systems: A critical review of theranostic behavior in cancer. Pharmaceutics. 2021;13(3):355
12. Jain A, Hurkat P, Jain SK. Development of liposomes using formulation by design: Basics to recent advances. Chemistry and physics of lipids. 2019;224:104764
13. Li M, Du C, Guo N, Teng Y, Meng X, Sun H. et al. Composition design and medical application of liposomes. European journal of medicinal chemistry. 2019;164:640-53
14. Paolino D, Cosco D, Gaspari M, Celano M, Wolfram J, Voce P. et al. Targeting the thyroid gland with thyroid-stimulating hormone (TSH)-nanoliposomes. Biomaterials. 2014;35(25):7101-9
15. Brown BS, Patanam T, Mobli K, Celia C, Zage PE, Bean AJ. et al. Etoposide-loaded immunoliposomes as active targeting agents for GD2-positive malignancies. Cancer biology & therapy. 2014;15(7):851-61
16. Wang J, Gong J, Wei Z. Strategies for Liposome Drug Delivery Systems to Improve Tumor Treatment Efficacy. AAPS PharmSciTech. 2022;23(1):1-14
17. Trucillo P, Campardelli R, Iuorio S, De Stefanis P, Reverchon E. Economic analysis of a new business for liposome manufacturing using a high-pressure system. Processes. 2020;8(12):1604
18. Chauhan SB, Gupta V. Recent advances in liposome. Research Journal of Pharmacy and Technology. 2020;13(4):2053-8
19. Pasut G, Paolino D, Celia C, Mero A, Joseph AS, Wolfram J. et al. Polyethylene glycol (PEG)-dendron phospholipids as innovative constructs for the preparation of super stealth liposomes for anticancer therapy. Journal of controlled release. 2015;199:106-13
20. De Pauw B. Fungal infections: diagnostic problems and choice of therapy. Leukemia supplements. 2012;1(2):S22-S3
21. Moosavian SA, Sahebkar A. Aptamer-functionalized liposomes for targeted cancer therapy. Cancer letters. 2019;448:144-54
22. Jain A, Tiwari A, Verma A, Jain SK. Ultrasound-based triggered drug delivery to tumors. Drug delivery and translational research. 2018;8(1):150-64
23. Jain A, Jain SK. Stimuli-responsive smart liposomes in cancer targeting. Current drug targets. 2018;19(3):259-70
24. Riaz MK, Riaz MA, Zhang X, Lin C, Wong KH, Chen X. et al. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. International journal of molecular sciences. 2018;19(1):195
25. Paliwal SR, Paliwal R, Agrawal GP, Vyas SP. Hyaluronic acid modified pH-sensitive liposomes for targeted intracellular delivery of doxorubicin. Journal of liposome research. 2016;26(4):276-87
26. Lee W, Im H-J. Theranostics based on liposome: looking back and forward. Nuclear Medicine and Molecular Imaging. 2019;53(4):242-6
27. Tu AB, Lewis JS. Biomaterial-based immunotherapeutic strategies for rheumatoid arthritis. Drug Delivery and Translational Research. 2021;11(6):2371-93
28. Chen CZ, Cooper SL. Recent advances in antimicrobial dendrimers. Advanced Materials. 2000;12(11):843-6
29. Wolinsky JB, Grinstaff MW. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Advanced drug delivery reviews. 2008;60(9):1037-55
30. Khemtong C, Kessinger CW, Gao J. Polymeric nanomedicine for cancer MR imaging and drug delivery. Chemical Communications. 2009(24):3497-510.
31. Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. Journal of cancer research and clinical oncology. 2015;141(5):769-84
32. Gupta U, Dwivedi SKD, Bid HK, Konwar R, Jain N. Ligand anchored dendrimers based nanoconstructs for effective targeting to cancer cells. International journal of pharmaceutics. 2010;393(1-2):186-97
33. Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nature materials. 2013;12(11):991-1003
34. Lai P-S, Lou P-J, Peng C-L, Pai C-L, Yen W-N, Huang M-Y. et al. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. Journal of Controlled Release. 2007;122(1):39-46
35. Hao Y, Gao Y, Fan Y, Zhang C, Zhan M, Cao X. et al. A tumor microenvironment-responsive poly (amidoamine) dendrimer nanoplatform for hypoxia-responsive chemo/chemodynamic therapy. Journal of Nanobiotechnology. 2022;20(1):1-15
36. Mu W, Chu Q, Liu Y, Zhang N. A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nano-Micro Letters. 2020;12(1):1-24
37. Fan W, Zhang L, Li Y, Wu H. Recent progress of crosslinking strategies for polymeric micelles with enhanced drug delivery in cancer therapy. Current medicinal chemistry. 2019;26(13):2356-76
38. Alven S, Aderibigbe BA. The therapeutic efficacy of dendrimer and micelle formulations for breast cancer treatment. Pharmaceutics. 2020;12(12):1212
39. Seo SJ, Lee SY, Choi SJ, Kim HW. Tumor-Targeting Co-Delivery of Drug and Gene from Temperature-Triggered Micelles. Macromolecular Bioscience. 2015;15(9):1198-204
40. Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacology & therapeutics. 2006;112(3):630-48
41. Wan X, Beaudoin JJ, Vinod N, Min Y, Makita N, Bludau H. et al. Co-delivery of paclitaxel and cisplatin in poly (2-oxazoline) polymeric micelles: Implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials. 2019;192:1-14
42. Razzaq S, Rauf A, Raza A, Akhtar S, Tabish TA, Sandhu MA. et al. A multifunctional polymeric micelle for targeted delivery of paclitaxel by the inhibition of the P-Glycoprotein transporters. Nanomaterials. 2021;11(11):2858
43. Ahmed W, Elhissi A, Dhanak V, Subramani K. Carbon nanotubes: Applications in cancer therapy and drug delivery research. Emerging Nanotechnologies in Dentistry: Elsevier. 2018 p. 371-89
44. Son KH, Hong JH, Lee JW. Carbon nanotubes as cancer therapeutic carriers and mediators. International journal of nanomedicine. 2016;11:5163
45. Kiran AR, Kumari GK, Krishnamurthy PT. Carbon nanotubes in drug delivery: Focus on anticancer therapies. Journal of Drug Delivery Science and Technology. 2020;59:101892
46. Yang F, Jin C, Yang D, Jiang Y, Li J, Di Y. et al. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. European Journal of Cancer. 2011;47(12):1873-82
47. Karimi M, Solati N, Ghasemi A, Estiar MA, Hashemkhani M, Kiani P. et al. Carbon nanotubes part II: a remarkable carrier for drug and gene delivery. Expert opinion on drug delivery. 2015;12(7):1089-105
48. Guo C, Al-Jamal WT, Toma FM, Bianco A, Prato M, Al-Jamal KT. et al. Design of cationic multiwalled carbon nanotubes as efficient siRNA vectors for lung cancer xenograft eradication. Bioconjugate chemistry. 2015;26(7):1370-9
49. Varkouhi AK, Foillard S, Lammers T, Schiffelers RM, Doris E, Hennink WE. et al. SiRNA delivery with functionalized carbon nanotubes. International journal of pharmaceutics. 2011;416(2):419-25
50. Niu Q, Lv W, Yan T, Wang J, Yan B, Zhou DJBM. Construction of Durvalumab/carbon nanotube/PEI/aptamer-siRNA chimera for the immunotherapy of hepatocellular carcinoma. 2022.
51. Fan H, Zhang I, Chen X, Zhang L, Wang H, Da Fonseca A. et al. Intracerebral CpG immunotherapy with carbon nanotubes abrogates growth of subcutaneous melanomas in mice. Clinical Cancer Research. 2012;18(20):5628-38
52. Jin H, Gao S, Song D, Liu Y, Chen X. Intratumorally CpG immunotherapy with carbon nanotubes inhibits local tumor growth and liver metastasis by suppressing the epithelial-mesenchymal transition of colon cancer cells. Anti-Cancer Drugs. 2021;32(3):278
53. Paul W, Sharma CP. Inorganic nanoparticles for targeted drug delivery. Biointegration of medical implant materials. 2020:333-73
54. Llevot A, Astruc D. Applications of vectorized gold nanoparticles to the diagnosis and therapy of cancer. Chemical Society Reviews. 2012;41(1):242-57
55. Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. Journal of the Chemical Society, Chemical Communications. 1994(7):801-2.
56. Lim Z-ZJ, Li J-EJ, Ng C-T, Yung L-YL, Bay B-H. Gold nanoparticles in cancer therapy. Acta Pharmacologica Sinica. 2011;32(8):983-90
57. Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. small. 2008;4(1):26-49
58. Li W, Cao Z, Liu R, Liu L, Li H, Li X. et al. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artificial cells, nanomedicine, and biotechnology. 2019;47(1):4222-33
59. Wang F, Li C, Cheng J, Yuan Z. Recent advances on inorganic nanoparticle-based cancer therapeutic agents. International journal of environmental research and public health. 2016;13(12):1182
60. Haume K, Rosa S, Grellet S, Śmiałek MA, Butterworth KT, Solov'yov AV. et al. Gold nanoparticles for cancer radiotherapy: a review. Cancer nanotechnology. 2016;7(1):1-20
61. Nath R, Bongiorni P, Rockwell S. Iododeoxyuridine radiosensitization by low-and high-energy photons for brachytherapy dose rates. Radiation research. 1990;124(3):249-58
62. Matsudaira H, Ueno AM, Furuno I. Iodine contrast medium sensitizes cultured mammalian cells to X rays but not to γ rays. Radiation research. 1980;84(1):144-8
63. Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Physics in Medicine & Biology. 2004;49(18):N309
64. Yang C, Bromma K, Ciano-Oliveira D, Zafarana G, van Prooijen M, Chithrani DB. Gold nanoparticle mediated combined cancer therapy. Cancer Nanotechnology. 2018;9(1):1-14
65. Yang C, Bromma K, Sung W, Schuemann J, Chithrani D. Determining the radiation enhancement effects of gold nanoparticles in cells in a combined treatment with cisplatin and radiation at therapeutic megavoltage energies. Cancers. 2018;10(5):150
66. Chithrani DB, Jelveh S, Jalali F, Van Prooijen M, Allen C, Bristow RG. et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiation research. 2010;173(6):719-28
67. Wolfe T, Chatterjee D, Lee J, Grant JD, Bhattarai S, Tailor R. et al. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11(5):1277-83
68. Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. International journal of molecular sciences. 2018;19(7):1979
69. Ajnai G, Chiu A, Kan T, Cheng C-C, Tsai T-H, Chang J. Trends of gold nanoparticle-based drug delivery system in cancer therapy. Journal of Experimental & Clinical Medicine. 2014;6(6):172-8
70. Kong F-Y, Zhang J-W, Li R-F, Wang Z-X, Wang W-J, Wang W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22(9):1445
71. Pawar HR, Bhosale SS, Derle ND. Use of liposomes in cancer therapy: A review. International Journal of Pharmaceutical Sciences and Research. 2012;3(10):3585
72. Xin J, Wang S, Wang B, Wang J, Wang J, Zhang L. et al. AlPcS4-PDT for gastric cancer therapy using gold nanorod, cationic liposome, and Pluronic® F127 nanomicellar drug carriers. International journal of nanomedicine. 2018;13:2017
73. Wu T, Duan X, Hu C, Wu C, Chen X, Huang J. et al. Synthesis and characterization of gold nanoparticles from Abies spectabilis extract and its anticancer activity on bladder cancer T24 cells. 2019;47(1):512-23.
74. Wu T, Duan X, Hu C, Wu C, Chen X, Huang J. et al. Synthesis and characterization of gold nanoparticles from Abies spectabilis extract and its anticancer activity on bladder cancer T24 cells. Artificial cells, nanomedicine, and biotechnology. 2019;47(1):512-23
75. Duncan B, Kim C, Rotello VM. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. Journal of controlled release. 2010;148(1):122-7
76. Chen W, Bardhan R, Bartels M, Perez-Torres C, Pautler RG, Halas NJ. et al. A molecularly targeted theranostic probe for ovarian cancer. Molecular cancer therapeutics. 2010;9(4):1028-38
77. Rodriguez-Leon E, Iniguez-Palomares R, Navarro RE, Herrera-Urbina R, Tanori J, Iniguez-Palomares C. et al. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res Lett. 2013 8
78. Shu MJ, He FJ, Li ZH, Zhu XZ, Ma YJ, Zhou ZH. et al. Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Yeast Extract as Reducing and Capping Agents. Nanoscale Res Lett. 2020 15(1)
79. Dauthal P, Mukhopadhyay M. In-vitro free radical scavenging activity of biosynthesized gold and silver nanoparticles using Prunus armeniaca (apricot) fruit extract. J Nanopart Res. 2013 15(1)
80. Raza S, Yan W, Stenger N, Wubs M, Mortensen NA. Blueshift of the surface plasmon resonance in silver nanoparticles: substrate effects. Opt Express. 2013;21(22):27344-55
81. Sanchez-Moreno P, Ortega-Vinuesa JL, Peula-Garcia JM, Marchal JA, Boulaiz H. Smart Drug-Delivery Systems for Cancer Nanotherapy. Curr Drug Targets. 2018;19(4):339-59
82. Nikolova MP, Chavali MS. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics-Basel. 2020 5(2)
83. Chen F, Hong H, Shi S, Goel S, Valdovinos HF, Hernandez R. et al. Engineering of hollow mesoporous silica nanoparticles for remarkably enhanced tumor active targeting efficacy. Scientific reports. 2014;4(1):1-10
84. Yang S, Chen D, Li N, Xu Q, Li H, Gu F. et al. Hollow mesoporous silica nanocarriers with multifunctional capping agents for in vivo cancer imaging and therapy. Small. 2016;12(3):360-70
85. Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J. et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11(2):313-27
86. Barabadi H, Vahidi H, Mahjoub MA, Kosar Z, Damavandi Kamali K, Ponmurugan K. et al. Emerging antineoplastic gold nanomaterials for cervical cancer therapeutics: a systematic review. Journal of Cluster Science. 2020;31(6):1173-84
87. Chan M-H, Lin H-M. Preparation and identification of multifunctional mesoporous silica nanoparticles for in vitro and in vivo dual-mode imaging, theranostics, and targeted tracking. Biomaterials. 2015;46:149-58
88. Möller K, Bein T. Talented mesoporous silica nanoparticles. Chemistry of Materials. 2017;29(1):371-88
89. Liu Q, Xia W. Mesoporous silica nanoparticles for cancer therapy. New Advances on Disease Biomarkers and Molecular Targets in Biomedicine: Springer. 2013 p. 231-42
90. Xu C, Chen F, Valdovinos HF, Jiang D, Goel S, Yu B. et al. Bacteria-like mesoporous silica-coated gold nanorods for positron emission tomography and photoacoustic imaging-guided chemo-photothermal combined therapy. Biomaterials. 2018;165:56-65
91. Li T, Shen X, Geng Y, Chen Z, Li L, Li S. et al. Folate-functionalized magnetic-mesoporous silica nanoparticles for drug/gene codelivery to potentiate the antitumor efficacy. ACS Applied Materials & Interfaces. 2016;8(22):13748-58
92. Yang H, Chen Y, Chen Z, Geng Y, Xie X, Shen X. et al. Chemo-photodynamic combined gene therapy and dual-modal cancer imaging achieved by pH-responsive alginate/chitosan multilayer-modified magnetic mesoporous silica nanocomposites. Biomaterials science. 2017;5(5):1001-13
93. Zhao S, Xu M, Cao C, Yu Q, Zhou Y, Liu J. A redox-responsive strategy using mesoporous silica nanoparticles for co-delivery of siRNA and doxorubicin. Journal of Materials Chemistry B. 2017;5(33):6908-19
94. Chen L, She X, Wang T, Shigdar S, Duan W, Kong L. Mesoporous silica nanorods toward efficient loading and intracellular delivery of siRNA. Journal of Nanoparticle Research. 2018;20(2):1-13
95. Li X, Chen Y, Wang M, Ma Y, Xia W, Gu H. A mesoporous silica nanoparticle-PEI-fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials. 2013;34(4):1391-401
96. Wang Z, Chang Z, Lu M, Shao D, Yue J, Yang D. et al. Shape-controlled magnetic mesoporous silica nanoparticles for magnetically-mediated suicide gene therapy of hepatocellular carcinoma. Biomaterials. 2018;154:147-57
97. Liu X, Su H, Shi W, Liu Y, Sun Y, Ge D. Functionalized poly (pyrrole-3-carboxylic acid) nanoneedles for dual-imaging guided PDT/PTT combination therapy. Biomaterials. 2018;167:177-90
98. Yuan Y, He Y, Bo R, Ma Z, Wang Z, Dong L. et al. A facile approach to fabricate self-assembled magnetic nanotheranostics for drug delivery and imaging. Nanoscale. 2018;10(46):21634-9
99. Balk M, Haus T, Band J, Unterweger H, Schreiber E, Friedrich R. et al. Cellular SPION Uptake and Toxicity in Various Head and Neck Cancer Cell Lines. Nanomaterials 2021, 11, 726. s Note: MDPI stays neutral with regard to jurisdictional claims in published …. 2021
100. Solar P, González G, Vilos C, Herrera N, Juica N, Moreno M. et al. Multifunctional polymeric nanoparticles doubly loaded with SPION and ceftiofur retain their physical and biological properties. Journal of nanobiotechnology. 2015;13(1):1-12
101. Luzier WD. Materials derived from biomass/biodegradable materials. Proceedings of the National Academy of Sciences. 1992;89(3):839-42
102. Liu Q, Cai C, Dong CM. Poly (l-lactide)-b-poly (ethylene oxide) copolymers with different arms: Hydrophilicity, biodegradable nanoparticles, in vitro degradation, and drug-release behavior. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2009;88(4):990-9
103. Tsukada Y, Hara K, Bando Y, Huang C, Kousaka Y, Kawashima Y. et al. Particle size control of poly (dl-lactide-co-glycolide) nanospheres for sterile applications. International journal of pharmaceutics. 2009;370(1-2):196-201
104. Park EK, Kim SY, Lee SB, Lee YM. Folate-conjugated methoxy poly (ethylene glycol)/poly (ɛ-caprolactone) amphiphilic block copolymeric micelles for tumor-targeted drug delivery. Journal of Controlled Release. 2005;109(1-3):158-68
105. Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annual review of biomedical engineering. 2012;14:1-16
106. Liang R, Wei M, Evans DG, Duan X. Inorganic nanomaterials for bioimaging, targeted drug delivery and therapeutics. Chemical Communications. 2014;50(91):14071-81
107. Kang T, Li F, Baik S, Shao W, Ling D, Hyeon T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials. 2017;136:98-114
108. Zou P, Yu Y, Wang YA, Zhong Y, Welton A, Galbán C. et al. Superparamagnetic iron oxide nanotheranostics for targeted cancer cell imaging and pH-dependent intracellular drug release. Molecular pharmaceutics. 2010;7(6):1974-84
109. Zhi D, Zhao Y, Cui S, Chen H, Zhang S. Conjugates of small targeting molecules to non-viral vectors for the mediation of siRNA. Acta biomaterialia. 2016;36:21-41
110. Zhao M-X, Zeng E-Z. Application of functional quantum dot nanoparticles as fluorescence probes in cell labeling and tumor diagnostic imaging. Nanoscale research letters. 2015;10(1):1-9
111. Lu Y, Zhong Y, Wang J, Su Y, Peng F, Zhou Y. et al. Aqueous synthesized near-infrared-emitting quantum dots for RGD-based in vivo active tumour targeting. Nanotechnology. 2013;24(13):135101
112. Ghasemi Y, Peymani P, Afifi S. Quantum dot: magic nanoparticle for imaging, detection and targeting. Acta Biomed. 2009;80(2):156-65
113. Liu L, Yong K-T, Roy I, Law W-C, Ye L, Liu J. et al. Bioconjugated pluronic triblock-copolymer micelle-encapsulated quantum dots for targeted imaging of cancer: in vitro and in vivo studies. Theranostics. 2012;2(7):705
114. Mashinchian O, Johari-Ahar M, Ghaemi B, Rashidi M, Barar J, Omidi Y. Impacts of quantum dots in molecular detection and bioimaging of cancer. BioImpacts: BI. 2014;4(3):149
115. Şenel B, Demir N, Büyükköroğlu G, Yıldız M. Graphene quantum dots: Synthesis, characterization, cell viability, genotoxicity for biomedical applications. Saudi Pharmaceutical Journal. 2019;27(6):846-58
116. Akbarzadeh M, Babaei M, Abnous K, Taghdisi SM, Peivandi MT, Ramezani M. et al. Hybrid silica-coated Gd-Zn-Cu-In-S/ZnS bimodal quantum dots as an epithelial cell adhesion molecule targeted drug delivery and imaging system. International journal of pharmaceutics. 2019;570:118645
117. Iannazzo D, Pistone A, Salamò M, Galvagno S, Romeo R, Giofré SV. et al. Graphene quantum dots for cancer targeted drug delivery. International journal of pharmaceutics. 2017;518(1-2):185-92
118. Javanbakht S, Namazi H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Materials Science and Engineering: C. 2018;87:50-9
119. Nasrollahi F, Koh YR, Chen P, Varshosaz J, Khodadadi AA, Lim S. Targeting graphene quantum dots to epidermal growth factor receptor for delivery of cisplatin and cellular imaging. Materials Science and Engineering: C. 2019;94:247-57
120. Su X, Chan C, Shi J, Tsang M-K, Pan Y, Cheng C. et al. A graphene quantum dot@ Fe3O4@ SiO2 based nanoprobe for drug delivery sensing and dual-modal fluorescence and MRI imaging in cancer cells. Biosensors and Bioelectronics. 2017;92:489-95
121. Singh MR, Chandra Sekhar M, Balakrishnan S, Masood S. Medical applications of hybrids made from quantum emitter and metallic nanoshell. Journal of Applied Physics. 2017;122(3):034306
122. Kim J-H, Kim Y-S, Park K, Lee S, Nam HY, Min KH. et al. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. Journal of Controlled Release. 2008;127(1):41-9
123. Liang Z, Li X, Xie Y, Liu S. 'Smart'gold nanoshells for combined cancer chemotherapy and hyperthermia. Biomedical Materials. 2014;9(2):025012
124. Zhao N, You J, Zeng Z, Li C, Zu Y. An ultra pH-sensitive and aptamer-equipped nanoscale drug-delivery system for selective killing of tumor cells. Small. 2013;9(20):3477-84
125. Pondman KM, Bunt ND, Maijenburg AW, van Wezel RJ, Kishore U, Abelmann L. et al. Magnetic drug delivery with FePd nanowires. Journal of Magnetism and Magnetic Materials. 2015;380:299-306
126. Peng F, Su Y, Wei X, Lu Y, Zhou Y, Zhong Y. et al. Silicon-Nanowire-Based Nanocarriers with Ultrahigh Drug-Loading Capacity for. Vitro.
127. Li K-C, Chu H-C, Lin Y, Tuan H-Y, Hu Y-C. PEGylated copper nanowires as a novel photothermal therapy agent. ACS applied materials & interfaces. 2016;8(19):12082-90
128. Wu G, Datar RH, Hansen KM, Thundat T, Cote RJ, Majumdar A. Bioassay of prostate-specific antigen (PSA) using microcantilevers. Nature biotechnology. 2001;19(9):856-60
129. Chen J, Tan Q, Yang Z, Jin Y. Engineered extracellular vesicles: potentials in cancer combination therapy. Journal of Nanobiotechnology. 2022;20(1):1-22
130. Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sinica. 2017;38(6):754-63
131. Vázquez-Ríos AJ, Molina-Crespo Á, Bouzo BL, López-López R, Moreno-Bueno G, de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. Journal of nanobiotechnology. 2019;17(1):1-15
132. Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C. et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature medicine. 2001;7(3):297-303
133. Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle-and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. Journal of Controlled Release. 2015;220:727-37
134. Li Y-J, Wu J-Y, Wang J-M, Hu X-B, Cai J-X, Xiang D-X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta biomaterialia. 2020;101:519-30
135. Emam SE, Lila ASA, Elsadek NE, Ando H, Shimizu T, Okuhira K. et al. Cancer cell-type tropism is one of crucial determinants for the efficient systemic delivery of cancer cell-derived exosomes to tumor tissues. European Journal of Pharmaceutics and Biopharmaceutics. 2019;145:27-34
136. Kim SM, Yang Y, Oh SJ, Hong Y, Seo M, Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. Journal of Controlled Release. 2017;266:8-16
137. Gomari H, Moghadam MF, Soleimani M, Ghavami M, Khodashenas S. Targeted delivery of doxorubicin to HER2 positive tumor models. International journal of nanomedicine. 2019;14:5679
138. Parfejevs V, Sagini K, Buss A, Sobolevska K, Llorente A, Riekstina U. et al. Adult stem cell-derived extracellular vesicles in cancer treatment: opportunities and challenges. Cells. 2020;9(5):1171
139. Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Molecular Therapy. 2015;23(5):812-23
140. Kosztowski T, Zaidi HA, Quiñones-Hinojosa A. Applications of neural and mesenchymal stem cells in the treatment of gliomas. Expert review of anticancer therapy. 2009;9(5):597-612
141. Shojaei S, Hashemi SM, Ghanbarian H, Salehi M, Mohammadi-Yeganeh S. Effect of mesenchymal stem cells-derived exosomes on tumor microenvironment: tumor progression versus tumor suppression. Journal of Cellular Physiology. 2019;234(4):3394-409
142. Yeo RWY, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ. et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Advanced drug delivery reviews. 2013;65(3):336-41
143. Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N. et al. Human bone marrow-and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem cell research & therapy. 2015;6(1):1-20
144. Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E. et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. Journal of controlled release. 2014;192:262-70
145. Yin L, Liu X, Shi Y, Ocansey DKW, Hu Y, Li X. et al. Therapeutic advances of stem cell-derived extracellular vesicles in regenerative medicine. Cells. 2020;9(3):707
146. Sterzenbach U, Putz U, Low L-H, Silke J, Tan S-S, Howitt J. Engineered exosomes as vehicles for biologically active proteins. Molecular Therapy. 2017;25(6):1269-78
147. Hernández-Camarero P, Amezcua-Hernández V, Jiménez G, García MA, Marchal JA, Perán M. Clinical failure of nanoparticles in cancer: mimicking nature's solutions. Nanomedicine. 2020;15(23):2311-24
148. Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP. et al. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Molecular Therapy. 2013;21(1):101-8
149. Liu M, Hu Y, Chen G. The antitumor effect of gene-engineered exosomes in the treatment of brain metastasis of breast cancer. Frontiers in Oncology. 2020;10:1453
150. Zhang B, Bie Q, Wu P, Zhang J, You B, Shi H. et al. PGD2/PTGDR2 signaling restricts the self-renewal and tumorigenesis of gastric cancer. Stem cells. 2018;36(7):990-1003
151. Sun Z, Zhang J, Li J, Li M, Ge J, Wu P. et al. Roles of mesenchymal stem cell-derived exosomes in cancer development and targeted therapy. Stem Cells International. 2021. 2021
152. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557-63
153. Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. International journal of nanomedicine. 2019;14:2847
154. Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta biomaterialia. 2019;94:482-94
155. Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM. et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. Journal of translational medicine. 2005;3(1):1-8
156. Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S. et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. Journal of translational medicine. 2005;3(1):1-13
157. Veerman RE, Akpinar GG, Eldh M, Gabrielsson S. Immune cell-derived extracellular vesicles-functions and therapeutic applications. Trends in Molecular Medicine. 2019;25(5):382-94
158. Federici C, Shahaj E, Cecchetti S, Camerini S, Casella M, Iessi E. et al. Natural-killer-derived extracellular vesicles: Immune sensors and interactors. Frontiers in Immunology. 2020;11:262
159. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature immunology. 2008;9(5):503-10
160. Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annual review of immunology. 2013;31:413-41
161. Jong AY, Wu C-H, Li J, Sun J, Fabbri M, Wayne AS. et al. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. Journal of extracellular vesicles. 2017;6(1):1294368
162. Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH. et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7(10):2732
163. Oelsner S, Waldmann A, Billmeier A, Röder J, Lindner A, Ullrich E. et al. Genetically engineered CAR NK cells display selective cytotoxicity against FLT3-positive B-ALL and inhibit in vivo leukemia growth. International journal of cancer. 2019;145(7):1935-45
164. Yang P, Peng Y, Feng Y, Xu Z, Feng P, Cao J. et al. Immune Cell-Derived Extracellular Vesicles-New Strategies in Cancer Immunotherapy. Frontiers in immunology. 2021:5259.
165. Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunology letters. 2003;89(2-3):125-31
166. Xu Y, Liu Y, Yang C, Kang L, Wang M, Hu J. et al. Macrophages transfer antigens to dendritic cells by releasing exosomes containing dead-cell-associated antigens partially through a ceramide-dependent pathway to enhance CD 4+ T-cell responses. Immunology. 2016;149(2):157-71
167. Yang P, Cao X, Cai H, Feng P, Chen X, Zhu Y. et al. The exosomes derived from CAR-T cell efficiently target mesothelin and reduce triple-negative breast cancer growth. Cellular immunology. 2021;360:104262
168. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383-90
169. Li Y-J, Wu J-Y, Liu J, Xu W, Qiu X, Huang S. et al. Artificial exosomes for translational nanomedicine. Journal of nanobiotechnology. 2021;19(1):1-20
170. Deng Z, Rong Y, Teng Y, Mu J, Zhuang X, Tseng M. et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Molecular Therapy. 2017;25(7):1641-54
171. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Frontiers in pharmacology. 2015;6:286
172. Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E. et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Molecular Therapy. 2016;24(10):1783-96
173. Wang Q, Zhuang X, Mu J, Deng Z-B, Jiang H, Zhang L. et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nature communications. 2013;4(1):1-13
174. Wang Q, Ren Y, Mu J, Egilmez NK, Zhuang X, Deng Z. et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer research. 2015;75(12):2520-9
175. Amaro MI, Rocha J, Vila-Real H, Eduardo-Figueira M, Mota-Filipe H, Sepodes B. et al. Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Research International. 2009;42(8):1010-7
176. Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z. et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321-40
177. Raimondo S, Naselli F, Fontana S, Monteleone F, Dico AL, Saieva L. et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6(23):19514
178. Cooley M, Sarode A, Hoore M, Fedosov DA, Mitragotri S, Gupta AS. Influence of particle size and shape on their margination and wall-adhesion: implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale. 2018;10(32):15350-64
179. Ankamwar B. Size and shape effect on biomedical applications of nanomaterials. Biomedical Engineering-Technical Applications in Medicine. 2012:93-114
180. Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. 2008.
181. Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI. et al. Renal clearance of nanoparticles. Nature biotechnology. 2007;25(10):1165
182. Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano today. 2015;10(4):487-510
183. Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharmaceutical research. 2008;25(8):1815-21
184. Pacheco P, White D, Sulchek T. Effects of microparticle size and Fc density on macrophage phagocytosis. PloS one. 2013;8(4):e60989
185. Slack JD, Kanke M, Simmons GH, Deluca PP. Acute hemodynamic effects and blood pool kinetics of polystyrene microspheres following intravenous administration. Journal of pharmaceutical sciences. 1981;70(6):660-4
186. Muzykantov V, Muro S. Targeting delivery of drugs in the vascular system. International journal of transport phenomena. 2011;12(1-2):41
187. Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T. et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nature nanotechnology. 2007;2(4):249-55
188. Lee S-Y, Ferrari M, Decuzzi P. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. 2009;20(49):495101
189. Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Annals of biomedical engineering. 2005;33(2):179-90
190. Lai SK, Wang Y-Y, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced drug delivery reviews. 2009;61(2):158-71
191. Majcher MJ, Babar A, Lofts A, Leung A, Li X, Abu-Hijleh F. et al. In situ-gelling starch nanoparticle (SNP)/O-carboxymethyl chitosan (CMCh) nanoparticle network hydrogels for the intranasal delivery of an antipsychotic peptide. Journal of controlled release. 2021;330:738-52
Author contact
![]() Corresponding authors: ahmadian.elhamcom, dawid.janaspl, davaranac.ir
Corresponding authors: ahmadian.elhamcom, dawid.janaspl, davaranac.ir

 Global reach, higher impact
Global reach, higher impact