ISSN: 2206-7418
Nanotheranostics 2021; 5(1):1-7. doi:10.7150/ntno.47226 This issue Cite
Research Paper
Cancer-derived EVs show tropism for tissues at early stage of neoplastic transformation
1. Department of Health Sciences, Center of Excellence on Neurodegenerative Diseases, University of Milan, Italy.
2. Current address: Department of Pharmaceutical and Pharmacological Sciences, University of Padova, Italy.
3. Department of Pharmacological and Biomolecular Sciences, University of Milan, Milan, Italy.
4. Targovax Oy, Clinical Science, Helsinki, Finland.
5. National Institute of Public Health - National Institute of Hygiene, Department of Virology, Warsaw, Poland.
6. Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy.
7. Istituto Nazionale Tumori Fondazione IRCCS, National Cancer Institute, Milan, Italy.
#These authors contributed equally to this work.
Received 2020-4-27; Accepted 2020-8-21; Published 2021-1-1
Abstract
From the past decade, extracellular vesicles (EVs) have attracted considerable attention as tools for the selective delivery of anti-neoplastic drugs to cancer tissues. Compared to other nanoparticles, EVs display interesting unique features including immune compatibility, low toxicity and the ability to encapsulate a large variety of small- and macro-molecules. However, in virtually all studies, investigations on EVs have been focused on fully transformed cancers: the possibility to apply EV technology also to early-stage tumors has never been explored.
Methods: Herein, we studied the ability of cancer-derived EVs to recognize and deliver their cargo also to incipient cancers. To this purpose, EV biodistribution was studied in MMTV-NeuT genetically modified mice during early mammary transformation, in fully developed breast tumors and in the normal gland of wild type syngeneic mice. EVs were loaded with indocyanine green (ICG), a near-infrared (NIR) dye together with oncolytic viruses and i.v. injected in mice. The nanoparticle biodistribution was assayed by in vivo and ex vivo optical imaging (detecting the ICG) and semiquantitative real-time PCR (measuring the adenoviral genome) in different tissues.
Results: Our results demonstrate the ability of cancer-derived EVs to recognize early-stage neoplastic tissues opening the possibility to selectively deliver theranostics also for tumor prevention.
Conclusions: Taken together our study demonstrates the ability of EVs to recognize and deliver diagnostic and therapeutic agents not only to fully transformed tissues but also to early stage tumors. These findings pave the way for the synthesis of “universal” EVs-based formulation for targeted cancer therapy.
Keywords: Extracellular vesicles, oncolytic viruses, early-stage cancer therapy, drug delivery, in vivo imaging
Introduction
In the recent years, EVs, nanometer- to micron-sized lipid bilayered vesicles able to shuttle biological molecules over long distances [1] and have gained considerable attention as emerging tools for the delivery of theranostics agents [2-7]. EVs physiologically encapsulate metabolites, proteins or miRNA and have been artificially loaded with small molecule and biologics as therapeutic or diagnostic cargos, with the aim of obtaining their targeted delivery to specific cell populations. EVs encapsulation can improve drug pharmacokinetics providing a tissue-specific homing, protecting the delivered molecules from degradation and their early systemic release before reaching the target tissue [8-10]. Recent works provided compelling evidence of the tumor-selective homing of cancer-derived EVs [11-13]; this tropism is not specific for the tumor tissue originating the EVs, but it is maintained for different cancer types arising even from different species that might reflect an ancillary conserved mechanism of intercellular communication operating during tumor progression [14]. For these features, EVs are particularly attractive for the delivery of anti-neoplastic drugs, including small molecules and biologics [15], because they can direct the therapy selectively to the tumor site: a formulation-mediated targeted therapy with the potential to increase the local concentration of EVs-encapsulated drugs, while reducing systemic side effects. A current limitation of this formulation is that can be applied only for the treatment of advanced cancers, because demonstration of the homing capability of EVs for early cancer stage has not been provided yet. The possibility to deliver high dosages of diagnostics, chemo-preventive agents, chemotherapies or biopharmaceuticals locally, to the site of initial neoplastic transformation is an attractive strategy that is expected to exploit the anticancer effects of drugs on the emerging tumor with potentially relevant consequences on patients' survival. Hence, in the current study, we set to investigate whether the systemic administration of cancer derived EVs is able to deliver theranostics to early stage mammary cancers generated in a genetic mouse model of breast cancer (MMTV-NeuT mice) [16]. For these experiments, we encapsulated into EVs a diagnostic fluorescent dye [17] and an oncolytic virus [18-21] as tracking agents for the characterization of the whole-body biodistribution of the formulations. Herein, we show that cancer-derived EVs are able to target the mammary glands in MMTV-NeuT mice as soon as they start developing a neoplastic tissue, thus suggesting the possibility to deliver theranostic cargos of EVs also to early stage neoplasia.
Materials and Methods
Cell lines and virus
MC-38 mouse colon cancer and 4T1 mouse mammary tumor cell lines were purchased from the American Type Culture Collection (ATCC, USA). Cells were cultured at 37 °C and 5 % CO2 in Dulbecco's modified eagle medium (DMEM, Lonza, Switzerland) supplemented with 10% fetal bovine serum (FBS, Gibco Laboratories, USA), 1 % of 100 u/mL penicillin/streptomycin (Gibco Laboratories) and 1% L-glutamine (Gibco Laboratories). Oncolytic adenovirus Ad5D24 has been characterized by performing titration (VP/ml) and molecular analyses (PCR, restriction enzyme assay) to check virus genome stability and integrity as described elsewhere [22], expanded in human lung cancer cell line A549 and purified on cesium chloride gradients [23]. The viral particle concentration was determined by OD260-reading and standard TCID50 (tissue culture infectious dose 50) assay was performed to determine infectious particle titer.
Production of EV, EV-virus and ICG loaded EV formulations
In order to produce EVs, 2.6 x 106 MC-38 cells were plated into T-175 flask in medium supplemented with 5 % FBS. The FBS growth media was ultra-centrifuged overnight (110 000 x g at 4°C for 18 hours, Optima LE-80K ultracentrifuge, rotor type 50.2, Beckman Coulter) to remove EVs present in serum.
EVs were isolated from the conditioned medium using differential centrifugation steps as previously reported [9,11,12]. First the conditioned medium was centrifuged at 500 × g in 4°C for 10 minutes to pellet cells (Allegra X-15R Centrifuge, Beckman Coulter). Then, the supernatant was collected and ultra-centrifuged for 2 hours at 100 000 × g in 4°C, using Optima L-80 XP ultra-centrifuge (Beckman Coulter) with rotor SW32Ti (Beckman Coulter). The supernatant was aspirated and EV- containing pellets containing re-suspended in PBS (Lonza) 100 μL and stored at - 80 °C.
EV-encapsulated virus (EV-Virus) were produced as previously described, Virus samples were incubated in 100 mM NaOH at room temperature for 20 minutes in order to inactivate any free not EV encapsulated virus present. Free virus used as control was always inactivated for each experiment performed as previously reported [9]. Samples were subsequently neutralized by the addition of HCl 0.1 M.
EVs and EV-Virus were loaded with Indocyanine green (EV-ICG) and prepared by incubating 1×108 - 5×109 EVs in PBS for 12 h at 4°C with 10ug/mL ICG (Sigma). Next, the samples were centrifuged at 150 000 x g for 3 h to pellet the EVs. The supernatant containing unbound ICG was removed, and the EV-pellet was washed by suspending it in PBS and pelleting it again at 150 000 × g. The final EV-ICG-Virus pellet were re-suspended in 100 μL of PBS and stored at -80 °C until use. EV-formulations have been further characterized as previously reported [9,11,12,14].
Quantitative real-time PCR
qPCR for adenovirus E4 copy number was carried out according to the protocol previously described [24] primer forward: 5'-GGA GTG CGC CGA GAC AAC-3', primer reverse: 5'-ACT ACG TCC GGC GTT CCA T-3', probe E4: 5'-(6FAM)-TGG CAT GAC ACT ACG ACC AAC ACG ATC T- (TAMRA)-3'. Total DNA was extracted from resected brains, tumors, livers, blood from C57BL/6 mouse model after 24h post treatment, using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to manufacturer's protocol. Subsequently isolated DNA was analyzed for adenoviral E4 copy number normalized to murine beta-actin (liver, blood): primer forward: 5'-CGA GCG GTT CCG ATG C-3', primer reverse: 5'-TGG ATG CCA CAG GAT TCC AT-3', probe murine beta-actin: 5'-(6FAM)-AGG CTC TTT TCC AGC CTT CCT TCT TGG-(TAMRA)-3'. Samples were analyzed using LighCycler qPCR machine (LighCycler 480, Roche, Basel, Switzerland).
In vivo experiments and pharmacological treatments
All animal experimentations were performed and approved by the Italian Ministry of Research and University and controlled by a Departmental panel of experts (permission numbers: 12-12-30012012, 547/2015, 214/2020). MMTV-NeuT and C57Bl/6 mice were used for the experiments. The acclimatization period was 14 days prior MC-38 and 4T1 cancer cell injections in C57Bl/6 mice, health status of the engrafted mice was monitored daily and as soon as signs of pain or distress were evident, they were euthanized. For early cancer detection, female MMTV-NeuT mice were enrolled in the experiments at week 6 of age, when hyperplasia was evident and full transformation of the breast has not yet occurred [25]. EV-ICG-Virus-MC38 (n=5) (1×108 particles/injection + 1×108 viral particles/injection +10 ug/mL) were administered i.v in a volume of 100 µl.
In vivo and ex vivo imaging
In vivo and ex vivo fluorescence imaging has been carried out 24 hours post EV treatments using IVIS Lumina II Quantitative Fluorescent Imaging (PerkinElmer, Waltham, MA, USA) with suitable filters (Cy5.5) and following the manufacturer's instructions for fluorescence background subtraction. Mice were anaesthetized using Isofluorane (Isofluorane-Vet; Merial, Lyon, France) and kept under anesthesia during imaging sessions carried out with the Imaging System (5 min for dorsal view and 5 min for ventral view) (IVIS Lumina II Quantitative Fluorescent and Bioluminescent Imaging; PerkinElmer, Waltham, MA, USA). Photon emission in selected body areas was measured using the Living Image Software 3.2 (PerkinElmer). For the ex vivo imaging, mice were treated with luciferin 15 min prior euthanasia by cervical dislocation and ex vivo imaging of the selected organs were carried out immediately after death. The quantification was done with Living Image Software 3.2 (PerkinElmer).
Histopathological analysis
In order to perform histopathological evaluation, breast and tumor tissues explanted from MMTV-NeuT mice were immersed in a 4% formaldehyde solution for 24 hours in order to obtain chemical fixation. Samples were then dehydrated with ethanol, washed with xylene and included in paraffine; then they were cut into 4 and 10 µm thick sections for hematoxylin/eosin staining and fluorescence imaging, respectively. Slides were then analyzed with a confocal microscope (Nikon A1R laser scanning confocal microscope) to acquire the fluorescent signal released by ICG and compared with the adjacent hematoxylin/eosin sections acquired with a Virtual Slide Microscope (Olympus VS120). Throughout the process of fixing and paraffining, the samples were kept out of the light, in order to avoid the loss of the fluorescent signal. Based on our previous experiments carried out with a similar tumor model and in vivo imaging endpoints [11,12,14] and taken into account the experimental error, we calculated that five mice per experimental group were sufficient to assess the homing capabilities of EV-formulations in mice bearing hyperplastic or transformed mammary gland versus syngeneic healthy controls. Sampling and histology on the resected tumor specimens were determined by professional pathologists (at Pathology Department, Istituto Nazionale Tumori IRCCS Foundation, Milan, Italy). They were not blinded when assessing EV homing with in vivo imaging, while were blinded when assessing EV homing in histopathological samples. Statistical significance was assessed by using one-way ANOVA with Tukey's Multiple Comparison test and nonparametric Mann-Whitney test. All statistical analysis, calculations and tests were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA).
Results and Discussion
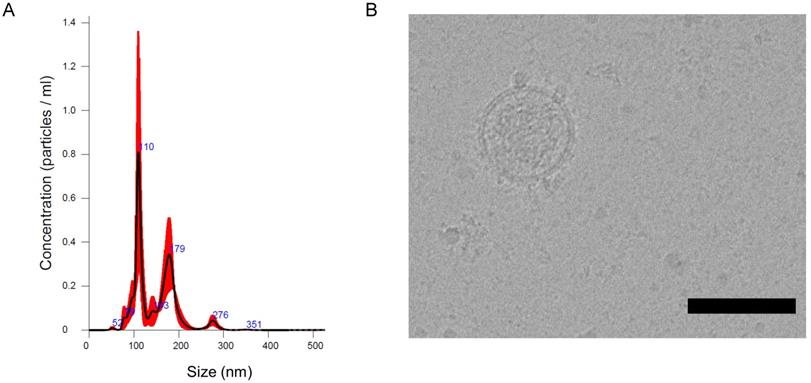
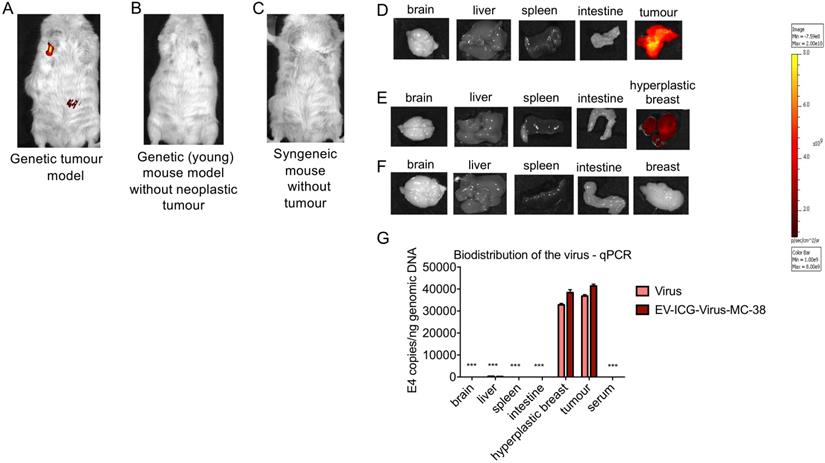
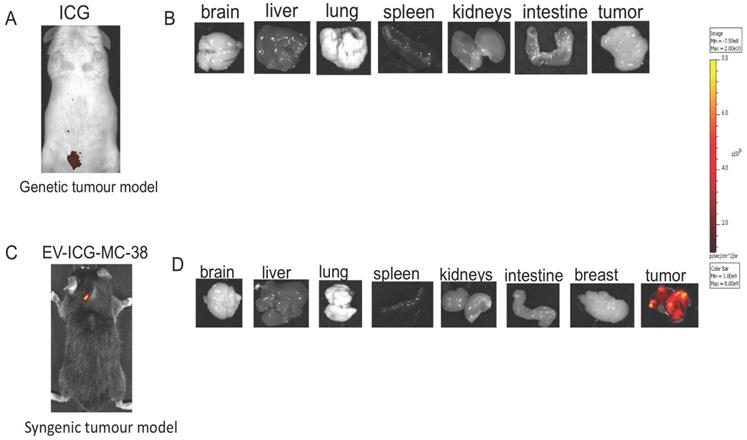
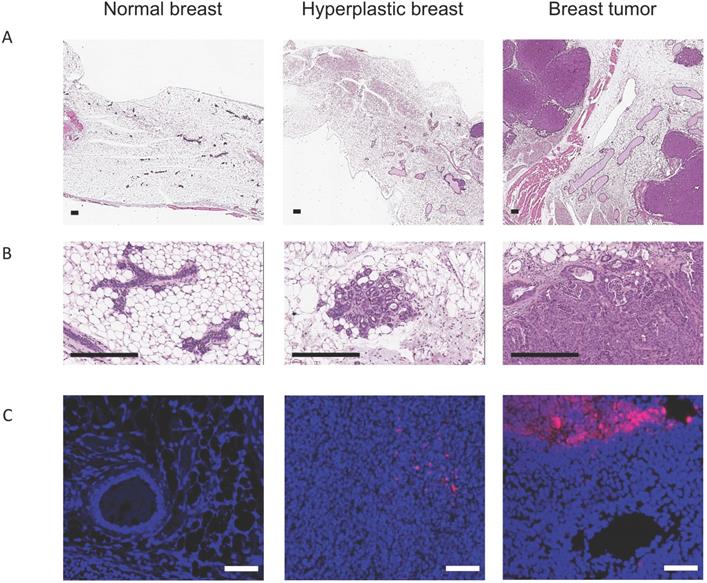
The recent work from our and other laboratories showed that tumor-derived EVs can be used to deliver diagnostics or therapeutics agents selectively to fully transformed tissues [12,14,9-27], while it was not clear the extent to which the tumor-tropism was applicable also to early-stage cancers. Herein, we studied the EV biodistribution in the MMTV-NeuT transgenic mouse, a genetic model of breast cancer [25], in which transformation of the mammary glands slowly progress from microscopic lesions detectable at weeks 6-8, to in situ neoplasia at weeks 9-15 until the appearance of a fully transformed invasive tumor usually arising after week 15 [16]. EVs from the MC-38 cancer cell line were prepared as previously described and displayed biophysical characteristics, in terms of size, distribution, and Zeta-potential, analogous to those produced in our previous work [14] (Figure 1A); as an additional test, cryo-EM experiments confirmed the EV size, morphology and integrity (Figure 1B). In order to track the destiny of cancer derived EV-Virus formulations within the body, we have loaded the biocompatible nanoparticles with ICG together with an oncolytic adenovirus (OA). The biodistribution was measured by in vivo and ex vivo fluorescence imaging and by semiquantitative real-time PCR to measure the amount of adenoviral genome in different tissues. MMTV-NeuT mice at 6 and 24 weeks of age and syngeneic controls were i.v. injected with the EV-ICG-Virus-MC38 formulations and 24 h post-treatment fluorescent emission was acquired by in vivo (Figure 2A,B,C) and ex vivo imaging (Figure 2D-F). Results showed that fluorescence emission originated mainly from the hyperplastic mammary gland (Figure 2A,D) or, to a greater extent, from the transformed breast of the MMTV-NeuT mice (Figure 2B,E), but not from the normal gland (Figure 2C,F). The qPCR analysis confirmed this conclusion showing that the OA content of the vesicles was delivered preferentially to the hyperplastic/neoplastic tissue of the MMTV-NeuT mice (Figure 2G). Interestingly, the tumor-specific tropism could not be detected when ICG was administered alone (Figure 3A-B) confirming that the fluorescence accumulation was due to the EV-mediated delivery of the dye. Furthermore, the EV-biodistribution in a syngeneic mouse model, subcutaneously injected with 4T1 murine breast cancer cells, showed a selective homing of EV-particles into the neoplastic tissue without targeting the mammary gland (Figure 3C-D). The preferential accumulation of the dye in the transformed breast was confirmed by confocal microscopy analysis performed on normal, hyperplastic and transformed breasts isolated from MMTV-NeuT and syngeneic mice (Figure 4). During the transition phase from pre-invasive to invasive ductal breast carcinoma, both mammary glands and their surrounding tissue experience extensive changes due to secretion of inflammatory factors by tumor cells, cancer-associated fibroblasts and macrophages. Such changes include a re-organization of the extracellular matrix and modifications in gene and protein expression profiles. Remarkably, tumoral EVs did recognize the pathological mammary glands independently from the stage: this observation suggests that the recognition mechanism driving the specific tropism of tumoral EVs could reflect these early changes occurring in the transforming tissue. Alternatively, it cannot be excluded that tumoral EVs present a repertoire of sensors allowing the migration towards tumoral microenvironments independently from the stage. Being the mechanism behind EV tropism still largely unknown, the reported results could offer a valuable hint for the identification of molecular determinants involved in the target recognition that should be conserved in both hyperplastic and fully transformed cells. Certainly, a thorough characterization of tumor-derived EVs is needed to fully exploit the great potential of these nanoparticles for diagnostic and therapeutic purposes.
Characterization of cancer derived EV-formulations (EV-ICG-Virus-MC-38). (A) Representative particle size distribution analysis of plasma-derived EVs obtained by nanoparticle tracking analysis (NTA). (B) EV imaging by cryo-electron microscopy, scale bar: 100 nm.
Cancer derived EV-formulations loaded with diagnostic and theranostics agents are able to target the tumor site and early phase neoplastic transformation. (A-C) Representative images of the whole-body photon emission (fluorescence) in MMTV-NeuT and syngeneic mice i.v treated with EV-ICG-Virus-MC-38. (D-F) Representative images of the photon emission in organs explanted from MMTV-NeuT and syngeneic mice. (G) Adenoviral copies towards E4 gene were measured by qPCR from euthanized mice's organs at the end of the treatment. Error bars mean+/- SD *p<0.05, **p<0.01, ***p<0.001.
Indocyanine green not loaded into EV-formulations cannot target the tumor site. (A-B) Representative images of the photon emission (fluorescence) in vivo and in organs explanted from MMTV-NeuT mice i.v. treated with ICG. (C-D) Representative images of the photon emission (fluorescence) in the tumor area and in organs explanted from syngeneic mice i.v. treated with EV-ICG-MC-38.
Whatever is the mechanism underlying the tumor selectivity, our data provide the rationale for novel potential clinical and diagnostical applications of EVs in the management of patients carrying early-stage cancers. We have previously demonstrated that the EV encapsulation prevents chemotherapeutic treatments to induce systemic inflammatory reactions, suggesting that EVs are able to deliver cytotoxic drugs to tumoral tissues to a sufficient amount for reaching therapeutic effects [9,12] and with a sufficient selectivity to avoid damages to other tissues [12]. Since a local high concentration of cytotoxic agents might be reached in this condition, our study pave the way for possible applications of chemotherapy in preventive clinical protocols aimed at the eradication of the disease before its full progression. Moreover, since EVs can be loaded with MRI or PET contrast agents [28,29], their high local concentration might boost the sensitivity of tumor detection, thus influencing the clinical management of patients carrying early-stage cancers.
Cancer derived EVs target neoplastic lesions. (A-B) Histopathological examination on normal breast, hyperplastic breast and breast tumour has been performed using Haematoxylin and Eosin staining (Scale bar: 2 mm). (C) Analysis of normal breast, hyperplastic breast and breast tumour by confocal microscopy. Nuclei stained with DAPI (blue), ICG signal in purple (Scale bar: 50 µm).
Despite these considerations, EV-mediated diagnostic and therapeutic applications are still in their infancy: a more comprehensive understanding of the role of EVs both in the progression of early and fully transformed cancer types is needed. Several issues need to be addressed before EVs use can be translated from the bench to clinical applications. Firstly, the methods for obtaining pure and intact EVs for routing clinical use need the application of reproducible isolation protocols that guarantee scale-up of sample volume, purity, speed, yield, and tumor specificity; finally, the clinical use of cancer-derived EVs certainly requires the clear definition of their possible side effects due to the potentially harmful cargo they are delivering together with the theranostic molecules.
Conclusions
Taken together the results of our study demonstrate the ability of EVs to recognize and deliver their diagnostic and therapeutic cargos not only to fully transformed tissues [11,12], but also to early stage tumors. Since chemotherapeutic drugs [30,31] as well as macromolecules or entire virus [9,32] and diagnostic agents for cancer imaging [28,29] can be encapsulated inside EVs, it is possible to envisage several applications in early-stage cancer diagnosis and therapy: high local concentration of PET or MRI contrast agents [33] might boost detection sensitivity while locally increasing the concentration of chemotherapy, have the potential to increase efficacy and greatly reduce side effects [12]. Still, some technical issue needs to be addressed for a reproducible and safe use of these tools including a standard, clinical grade purification protocol. The knowledge of the molecular determinants underlying their cancer-specific tropism will certainly facilitate their clinical translation by setting better purification protocols and perhaps in the future allowing the synthesis of “universal” EVs-based formulation for targeted cancer therapy.
Abbreviations
EVs: Extracellular Vesicles; MMTV-NeuT mice: genetic mouse model of breast cancer; ICG: Indocyanine green; EV-Virus: EV-encapsulated virus; EV-ICG: EV-encapsulated ICG; OA: oncolytic adenovirus.
Acknowledgements
This research was supported by the Italian Ministry of Education, University and Research under Grant “Departments of Excellence Italian Law n.232,11th December 2016” (P.C., V.M.); Italian Association for Cancer Research under Grant “IG-11903” (P.C.); by the Polish National Science Center (NCN) under Grant “Miniatura (2018/02/X/NZ7/00727) & SONATINA (2019/32/C/NZ7/00156).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI. et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:1-60
2. Gangadaran P, Li XJ, Kalimuthu S kumar, Min OJ, Hong CM, Rajendran RL. et al. New Optical Imaging Reporter-labeled Anaplastic Thyroid Cancer-Derived Extracellular Vesicles as a Platform for In vivo Tumor Targeting in a Mouse Model. Sci Rep. 2018;8:13509
3. Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J. et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698-7710
4. Jiang XC, Gao JQ. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521:167-175
5. Kooijmans SAA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: A novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525-1541
6. El Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-357
7. van der Meel R, Fens MHAM, Vader P, van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. J Control Release. 2014;195:72-85
8. Fais S, O'Driscoll L, Borras FE, Buzas E, Camussi G, Cappello F. et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano. 2016;10:3886-3899
9. Garofalo M, Saari H, Somersalo P, Crescenti D, Kuryk L, Aksela L. et al. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J Control Release. 2018;283:223-234
10. Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G. et al. Applying extracellular vesicles based therapeutics in clinical trials - An ISEV position paper. J Extracell Vesicles. 2015;4:1-31
11. Garofalo M, Villa A, Rizzi N, Kuryk L, Mazzaferro V, Ciana P. Systemic Administration and Targeted Delivery of Immunogenic Oncolytic Adenovirus Encapsulated in Extracellular Vesicles for Cancer Therapies. Viruses. 2018;10:558
12. Garofalo M, Villa A, Rizzi N, Kuryk L, Rinner B, Cerullo V. et al. Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J Control Release. 2019;294:165-175
13. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M. et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-335
14. Garofalo M, Villa A, Crescenti D, Marzagalli M, Kuryk L, Limonta P. et al. Theranostics Heterologous and cross-species tropism of cancer- derived extracellular vesicles. 2019; 9.
15. Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G. et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195-205
16. Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578-10582
17. Wang H, Li X, Tse BWC, Yang H, Thorling CA, Liu Y. et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics. 2018;8:1227-1242
18. Capasso C, Magarkar A, Cervera-Carrascon V, Fusciello M, Feola S, Muller M. et al. A novel in silico framework to improve MHC-I epitopes and break the tolerance to melanoma. Oncoimmunology. 2017;6:e1319028
19. Kuryk L, Møller A-SW, Garofalo M, Cerullo V, Pesonen S, Alemany R. et al. Anti-tumor specific T-cell responses induced by oncolytic adenovirus ONCOS-102 in peritoneal mesothelioma mouse model. J Med Virol. 2018:1-5
20. Cerullo V, Vähä-Koskela M, Hemminki A. Oncolytic adenoviruses: A potent form of tumor immunovirotherapy. Oncoimmunology. 2012;1:979-981
21. Kuryk L, Møller A-SW, Jaderberg M. Abscopal effect when combining oncolytic adenovirus and checkpoint inhibitor in a humanized NOG mouse model of melanoma. J Med Virol. 2019;91:1702-1706
22. Farzad L, Cerullo V, Yagyu S, Bertin T, Hemminki A, Rooney C. et al. Combinatorial treatment with oncolytic adenovirus and helper-dependent adenovirus augments adenoviral cancer gene therapy. Mol Ther - Oncolytics. 2014;1:14008
23. Kuryk L, Møller A-S, Vuolanto A, Pesonen S, Garofalo M, Cerullo V. et al. Optimization of Early Steps in Oncolytic Adenovirus ONCOS-401 Production in T-175 and HYPERFlasks. Int J Mol Sci. 2019;20:621
24. Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I. et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18:1874-84
25. Boggio BK, Nicoletti G, Carlo E Di, Cavallo F, Landuzzi L, Melani C. et al. Interleukin 12-mediated Prevention of Spontaneous Mammary Adenocarcinomas in Two Lines of Her-2/. 1998; 188.
26. van den Boorn JG, Schlee M, Coch C, Hartmann G. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29:325-326
27. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383-2390
28. Varga Z, Gyurkó I, Pálóczi K, Buzás EI, Horváth I, Hegedűs N. et al. Radiolabeling of Extracellular Vesicles with 99m Tc for Quantitative In vivo Imaging Studies. Cancer Biother Radiopharm. 2016;31:168-173
29. Shi S, Li T, Wen X, Wu SY, Xiong C, Zhao J. et al. Copper-64 Labeled PEGylated Exosomes for In vivo Positron Emission Tomography and Enhanced Tumor Retention. Bioconjug Chem. 2019;30:2675-2683
30. Jang SC, Kim OY, Yoon CM, Choi D-S, Roh T-Y, Park J. et al. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano. 2013;7:7698-7710
31. Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148-156
32. Ran L, Tan X, Li Y, Zhang H, Ma R, Ji T. et al. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials. 2016;89:56-66
33. Gangadaran P, Hong CM, Ahn B-C. An Update on in vivo Imaging of Extracellular Vesicles as Drug Delivery Vehicles. Front Pharmacol. 2018;9:169
Author contact
![]() Corresponding author: paolo.cianait; Tel.: +390250323030.
Corresponding author: paolo.cianait; Tel.: +390250323030.

 Global reach, higher impact
Global reach, higher impact