ISSN: 2206-7418
Nanotheranostics 2019; 3(2):196-211. doi:10.7150/ntno.34921 This issue Cite
Research Paper
Tumor-Activatable Clinical Nanoprobe for Cancer Imaging
1. Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, CA 90048
2. Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA 90048
3. S. Mark Taper Foundation Imaging Center, Cedars-Sinai Medical Center, Los Angeles, CA 90048
4. Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA 90048
5. Current Address: Department of Cell Biology and Biochemistry, Texas Tech University Health Sciences Center, Lubbock, TX, 79430
Abstract
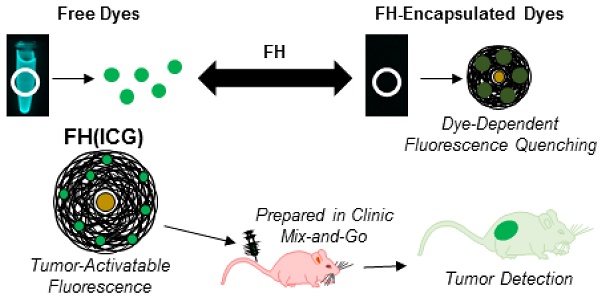
Purpose: A successful cancer surgery requires the complete removal of cancerous tissue, while also sparing as much healthy, non-cancerous tissue as possible. To achieve this, an accurate identification of tumor boundaries during surgery is critical, but intra-operative tumor visualization remains challenging. Fluorescence imaging is a promising method to improve tumor detection and delineate tumor boundaries during surgery, but the lack of stable, long-circulating, clinically-translatable fluorescent probes that can identify tumors with high signal-to-noise ratios and low background fluorescence signals have prevented its widespread application.
Methods: We screened the optical properties of several fluorescent dyes before and after nanoprobe encapsulation, and then identified nanoprobes with quenched fluorescence that were re-activated upon dye release. The physical and biological properties of these nanoprobes leading to fluorescence activation were investigated in vitro. Further, the cancer imaging properties of both free dyes and nanoprobe-encapsulated dyes were compared in vivo.
Results: A novel fluorescent nanoprobe was prepared by combining two FDA-approved agents commonly used in the clinic: Feraheme (FH) and indocyanine green (ICG). The resulting FH-entrapped ICG nanoprobe [FH(ICG)] displayed quenched fluorescence compared to other nanoprobes, and this quenched fluorescence was re-activated in acidic tumor microenvironment conditions (pH 6.8) and upon uptake into cancer cells. Finally, in vivo studies in a prostate cancer mouse model demonstrated that FH(ICG) treatments enhance long-term fluorescence signals in tumors compared to ICG treatments, allowing for fluorescence-guided tumor identification using clinically relevant fluorescence cameras.
Conclusions: FH(ICG) nanoprobes were identified as fluorescent nanoprobes with beneficial fluorescence activation properties compared to other FH-entrapped dyes. The activatable nature of this nanoprobe allows for a low background fluorescence signal and high signal-to-noise ratio within a long-circulating nanoagent, which allows for long-term fluorescence signals from tumors that enabled their fluorescence-guided detection. This activatable nanoprobe offers tremendous potential as a clinically translatable image-guided cancer therapy modality that can be prepared in a clinical setting.
Keywords: Indocyanine green, Feraheme, fluorescence imaging, image-guided surgery, prostate cancer

 Global reach, higher impact
Global reach, higher impact