ISSN: 2206-7418
Nanotheranostics 2017; 1(3):244-260. doi:10.7150/ntno.19796 This issue Cite
Review
Nanoparticle Vaccines Adopting Virus-like Features for Enhanced Immune Potentiation
1. Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan;
2. Taiwan International Graduate Program in Molecular Medicine, National Yang-Ming University and Academia Sinica, Taipei, Taiwan;
3. Department of Veterinary Medicine, National Taiwan University, Taipei, Taiwan;
4. Research Center for Nanotechnology and Infectious Diseases, Taipei, Taiwan.
Received 2017-2-24; Accepted 2017-4-17; Published 2017-6-9
Abstract
Synthetic nanoparticles play an increasingly significant role in vaccine design and development as many nanoparticle vaccines show improved safety and efficacy over conventional formulations. These nanoformulations are structurally similar to viruses, which are nanoscale pathogenic organisms that have served as a key selective pressure driving the evolution of our immune system. As a result, mechanisms behind the benefits of nanoparticle vaccines can often find analogue to the interaction dynamics between the immune system and viruses. This review covers the advances in vaccine nanotechnology with a perspective on the advantages of virus mimicry towards immune potentiation. It provides an overview to the different types of nanomaterials utilized for nanoparticle vaccine development, including functionalization strategies that bestow nanoparticles with virus-like features. As understanding of human immunity and vaccine mechanisms continue to evolve, recognizing the fundamental semblance between synthetic nanoparticles and viruses may offer an explanation for the superiority of nanoparticle vaccines over conventional vaccines and may spur new design rationales for future vaccine research. These nanoformulations are poised to provide solutions towards pressing and emerging human diseases.
Keywords: Nanoparticle vaccine, lymphatic delivery, repetitive antigen display, vaccine nanotechnology, cellular immunity, anticancer vaccine.
Introduction
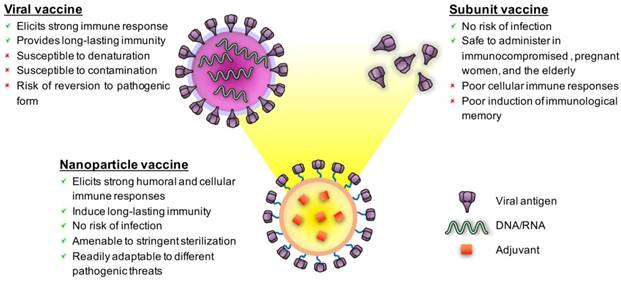
Vaccination is a process of introducing antigenic material to activate an individual's immune system to develop adaptive immunity to a pathogen. It has proven to be the most successful and cost-effective prophylactic measure against infectious diseases. Vaccines have been responsible for eradicating or effectively managing many major diseases, including smallpox, measles, mumps, rubella, diphtheria, tetanus, pertussis, polio, and yellow fever [1]. Despite the many examples of successful vaccines, many disease threats, such as human immunodeficiency virus (HIV), tuberculosis, dengue, and malaria, lack an effective prophylactic measure. Thus, development of new vaccine formulations and technology remains an ongoing quest [2]. Conventionally, vaccine formulations are comprised of biological materials in the form of attenuated viruses, killed pathogens, or subunit protein antigens. Each platform has its distinct advantages and shortcomings, frequently presenting a trade-off between safety and efficacy. For example, live attenuated vaccines are excellent at inducing long lasting protective immunity and strong immune response, but their “live” nature poses safety concerns, especially to individuals who may be immunocompromised. On the other hand, subunit vaccines are safer to administer, but they are less immunogenic and less effective at eliciting cellular immunity for disease protection (Fig. 1). Emerging technology and formulations to combine the advantages of live attenuated and subunit vaccines thus continue to be developed with the aim of maximizing vaccine safety and potency.
In the last decade, advances in materials engineering have opened up new avenues for innovative vaccine designs. In particular, synthetic nanoparticles have been widely adopted for vaccine development [3, 4]. These particles, typically 25 to 200 nm in diameter, have shown effective immune potentiation in vivo, capable of inducing strong humoral and cellular immune responses against antigen targets. Compared to live attenuated viral vaccines, synthetic nanoparticles promise better safety profiles because of their non-replicating nature. They are also readily amenable to different infectious pathogens [3]. From a holistic view, many advantages of nanoparticle vaccines may be attributed to their intrinsic semblance to natural viruses (Fig. 1). Many viral features, such as nanoscale morphology, repetitive multivalent antigen display and controlled antigen/adjuvant delivery are conducive to immune processing on both physiological and cellular levels. As our immune system has evolved to effectively respond to infectious viral nanoparticles, it should not come as a surprise that nanoformulations adopting virus-like features can be more potent than conventional subunit formulations.
To this date, numerous nanoparticle-based platforms have been examined for vaccine applications, and many have demonstrated encouraging efficacy against many pressing infectious threats, including malaria, influenza, Ebola, and HIV [5-8]. Also worth noting is the rapidly expanding field of nanoparticle-based anticancer vaccines, which exploits nanotechnology for enhanced induction of antigen-specific cellular immune responses against oncologic malignancies [9-12]. Many design considerations in nanoparticle vaccines can be traced to the fundamental interaction dynamics between viruses and the immune system [13]. Upon close scrutiny, virus-like mimicry in terms of size, geometry, antigen display, and adjuvanticity can be commonly observed among vaccine nanoformulations.
In light of the emerging landscape of nanoparticle vaccine research, this review highlights some of the underlying principles behind the advantages of nanoparticle vaccines. In the first section, commonly used biomaterials such as lipids, polymers and inorganic compounds are reviewed in the context of vaccine applications. In the sections that ensue, various nanoparticle design aspects are explored from a virus-mimetic perspective including lymphatic delivery, antigen display, and interaction with immune cells. Lastly, we highlight conjugation strategies that couple subunit protein antigens with nanocarriers. This review also summarizes the various strategies for conjugating antigens/adjuvants with nanocarriers. The review aims to provide a holistic view of the recent advances for the next generation of immunomodulatory vaccines.
Nanoparticles for vaccination
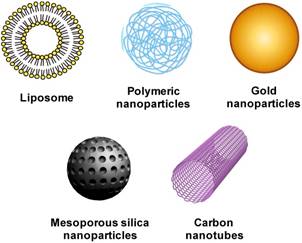
Towards mimicking viral features, preparing nanoparticulates in the range of 20 to 200 nm in size is the first step prior to subsequent vaccine development. It is therefore imperative to have an understanding of the various materials and their properties for nanoparticle preparations. The diverse range of nanomaterials for biomedical research encompasses a wide range of organic and inorganic matters, such as phospholipids, polymers, metal, silica, and carbon (Fig. 2). These nanomaterials can be modified to provide a functionalized and stable interface for different biomedical applications. In the context of vaccination, many nanocarriers have been modified to trigger specific immune responses analogous to natural defense mechanisms against viral invasion. The following section summarizes the different synthetic nanoparticles used in vaccine applications with an overview on their functionalizability and immunological adjuvanticity.
Schematics of a viral vaccine, a subunit vaccine, and a representative nanoparticle vaccine highlighting the strengths and shortcomings of each platform. Nanoparticle vaccines can be engineered to mimic viruses in terms of morphology, antigen display, and adjuvanticity.
Synthetic nanoparticles for vaccine delivery.
Liposomes and lipid-based nanoparticles
Liposomes are self-assembled, spherical vesicles consisting of a phospholipid bilayer and an aqueous inner core [14, 15]. They are prepared from phospholipids with fatty-acid chains of defined length and saturation. The choice of phospholipids and the addition of cholesterol influence liposomal stability and performance [16-18]. The vesicles can have a unilamellar structure with one layer of phospholipids or a multi-lamellar structure with several concentric phospholipid shells. This platform is highly versatile for cargoes delivery and allows for incorporation of hydrophilic molecules in the inner core and hydrophobic molecules within the phospholipid bilayers. Antigens and adjuvants may thus be incorporated into liposomes based on their lipophilicity [19, 20].
The lipid bilayered structure of liposomes is structurally analogous to enveloped viruses, which are formed from budding of infected cells and are wrapped in pieces of cell's plasma membrane. The inherent semblance to viral particles may help explain the innate adjuvanticity of liposomes upon incorporation with protein and peptide antigens [21]. Numerous antigen targets, ranging from toxoid, viral antigens, and bacterial antigens, have been observed to elicit enhanced humoral responses following liposomal incorporations [22]. Mechanistic studies have shed light on the liposomes' adjuvanticity. For instance, liposomes have been shown to be capable of modulating CD8+ T cell mediated immune responses [23], reflecting facilitation of antigen processing via the classical MHC I and MHC II pathways. Liposomes have also been reported to promote the development of T cell-independent B cell immune responses [24] and have potential to promote long-term immunity through the development of T-cell memory [25]. In addition to their innate adjuvanticity, liposomes have been incorporated with a plethora of adjuvants ranging from small molecules [26, 27], glycolipids [28-31], oligodeoxynucleotides [32-35], to cyclic dinucleotides [6, 36].
The structural fluidity of liposomes offers flexibility in platform modification towards virus mimicry. For example, the development of virosomes, which are fusion vesicles prepared from viral particles and synthetic liposomes, is an adaption that highlights the fundamental similarity between liposomes and viruses. Derived from the membrane vesicles of viruses, virosomes consist of liposome-like lipid vesicles and viral envelope glycoproteins. The fusion vesicles retain some viral characteristics (i.e. epitope presentation) and have been applied for both drug delivery and vaccination purposes [37, 38]. The applicability of this platform as a vaccine candidate has been demonstrated extensively using influenza virus-derived virosomes [39-42]. In studies that apply virosomes for anti-influenza vaccination, derivatized influenza virosomes were shown to be non-infective as their genetic materials were removed. Upon in vivo administration, these virosomes were rapidly uptaken by antigen presenting cells and in turn activated numerous other immune cells [43-46]. Immunization with virosomes was reported to reactivate influenza-specific memory CD4+ T cells that subsequently supported the proliferation of antigen-specific effector cells [46], leading to enhanced anti-influenza immune responses [47]. Besides influenza-based virosomes, induction of cytotoxic T lymphocyte responses has been demonstrated with a Sendai virus-based carrier system loaded with ovalbumin (OVA). It was demonstrated that Sendai virosomes fused with OVA elicited stronger CTL responses against the model antigen [48].
Liposomes may also be modified to enhance the stability of carriers in a manner analogous to how viruses employ viral matrix proteins to stabilize their lipid envelope [49]. In a study by Moon et. al., interbilayer-corsslinked multilamellar vesicles have been prepared by covalently crosslinking multiple layers of liposomes via thiol chemistry [50]. These multi-lamellar liposomes showed added stability owing to short covalent bonds that crosslinked adjacent lipid layers within the vesicle walls. This modification served to address some of the shortcomings of liposomes, facilitating enhanced antigen encapsulation and increased particle stability.
Also belonging to the class of lipid-based nanoparticles are an emerging class of lipoplexes, which consist of cationic lipid derivatives for the complexation with nucleic acids [10, 51, 52]. Immunostimulatory RNAs or mRNAs encoding specific antigen targets have been formulated into lipoplexes to trigger immune responses. The function of these lipoplexes can be likened to the immune response induction by RNA viruses [53]. As RNAs are delivered intracellularly by lipoplexes, they activate innate immune receptors, leading to an upregulation of type I interferons, which may further trigger a multitude of downstream immunological pathways [54]. Concurrently, these RNAs are translated into antigens of interest, thereby promoting an antigen-specific immune response. Lipoplexes carrying the mRNA of target antigens have recently been shown in a Phase I clinical trial to induce strong cellular responses against tumor antigens in humans [10].
Polymeric nanoparticles
A wide variety of polymers have been applied to the development of nanoparticle vaccines. Synthetic polymeric nanoparticles are typically solid particles between 10 nm to 200 nm. They have been an attractive platform for vaccine delivery as antigens and adjuvants can be either surface attached to or interior loaded inside these nanoparticles [55-59]. In particular, controlled release of biomolecules is one of the strongest advantage of polymeric nanoparticles, the release kinetics of which can be regulated by tuning of the copolymer composition and molecular weight [55]. Typically, polymeric nanoparticles are formed via self-assembly of amphiphilic copolymers under an emulsion or nanoprecipitation process [60-62]. Notable polymeric nanoparticles in vaccine development are as follows.
Poly(lactic-co-glycolic acid)(PLGA) is one of the most commonly used polymers for biomedical applications [63]. PLGA-based nanoparticles are known to be biodegradable, non-toxic and non-immunogenic. Upon administration, the polymer is degraded into lactic acids and glycolic acids in vivo to be safely metabolised in the body. In vaccine applications, PLGA nanoparticles provide a robust platform for antigen functionalization and have been used to carry antigen derived from various pathogens. Through surface conjugation or interior encapsulation, antigens including those derived from Plasmodium vivax [64], hepatitis B virus (HBV) [65], Bacillus anthracis [66], tetanus toxin [67], model antigens such as ovalbumin [68] have been associated with PLGA-based delivery systems for enhanced immune potentiation. Adjuvants have also been encapsulated or chemically attached to the PLGA polymer backbone for controlled delivery to enhance immune responses [69-72].
Natural polymers based on polysaccharide, such as pullulan [73-75], alginate [76], and chitosan [77-82], have been explored for nanoparticle vaccine preparations. In particular, chitosan-based nanoparticles have been widely studied due to their biocompatibility, biodegradability, non-toxic nature and their ability to be easily modified into desired shapes and sizes [83-85]. More intriguing is the recent discovery of chitosan's immune potentiating mechanism via the DNA sensing cGAS-STING pathway [86]. The STING pathway is triggered in the host cells by many viral pathogens and plays a major role in both the innate and adaptive immunity [87]. Upon interaction with dendritic cells, chitosan induces type I interferons in a cGAS and STING-dependent fashion, mediating cellular maturation and the promotion of Th1 responses. Chitosan-based nanoformulations have been widely adopted for vaccine development, examples of which include vaccines against Clostridium botulinum type-A neurotoxin [56], Neospora caninum [88], HBV [77], and Newcastle disease [81]. The polysaccharide polymer has also been applied to enhance the potency of DNA vaccines against viral, bacterial, and parasite infections [78, 80, 82].
Other polymeric nanoparticle platforms include poly(γ-glutamic acids)(γ-PGA) nanoparticles [83, 89], polystyrene nanoparticles [90, 91], and poly-alkyl acrylate based nanoparticles [92, 93]. γ-PGA are comprised of amphiphilic poly(amino acid)s, which self-assemble into nano-micelles with a hydrophilic outer shell and a hydrophobic inner core. They are generally used to encapsulate hydrophobic antigens. Polystyrene nanoparticles are solid particles consisting of polymerized styrene monomers that can be conjugated to a variety of antigens. Poly-alkyl acrylate based nanoformulations have been prepared with poly (methylmethacrylate) (PMMA), poly (ethylacrylic acid) (PEAA), poly (propylacrylic acid) (PPAA) and poly (butylacrylic acid) (PBAA). Studies have shown that polyacrylate-based nanoparticles show an inherent adjuvanticity with several model antigens [4, 94, 95]. The type of polymer used in the nano-formulation strongly affects the structure, properties and applications of the particles.
Gold nanoparticles
As gold is chemically inert, gold nanoparticles (AuNPs) have been studied extensively for biomedical applications. AuNPs can be synthesized reproducibly with a high level of precision, offering an ultrastable metallic core for further modifications. Capable of being modified with a plethora of chemical functional groups such as thiols, phosphines, amines, and by extension protein antigens, AuNPs have been used broadly in many vaccination studies. A multitude of biomolecules, ranging from toxin, viral antigens, bacterial antigens, parasite proteins, and tumor antigens, have been coupled with gold nanoparticles to enhance immune responses. AuNPs vaccines have been explored in clinical trials for hepatitis B and malaria vaccinations [96, 97], and they also allow anchoring of nucleic acids for DNA vaccine applications [98]. AuNPs have been modified with different adjuvants as well, such as chitosan [99] and CpG oligodeoxynucleotides [100, 101], and many alternative surface functionalization strategies have been explored to further enhance immune potentiation. AuNPs surface modified with cetyltrimethylammonium bromide (CTAB), poly(diallydimethylammonium chloride) (PDDAC), and polyethyleneimine (PEI), have been used as a DNA vaccine adjuvant for human immunodeficiency virus (HIV) [102]. These modifications were found to further boost the adjuvanticity of the AuNP carrier.
Gold's unique malleability makes AuNPs an attractive platform for vaccinology studies [103]. AuNPs can be fabricated into myriad of shapes (i.e. sphere, rod, cube, etc.) [104] with tunable yet sharply distributed size range between 2-250 nm [105]. This morphological tunability adds an additional dimension of virus mimicry with the introduction of non-spherical particles. Gold nanorods, for instance, have been used as a vaccine vector for the delivery respiratory syncytial virus (RSV) F protein [106]. These non-spherical particles bear resemblance to rod-like viruses that can also be frequently observed among different virus genera [107]. The size modularity of AuNPs also allows for examining the impact of particle size on vaccine delivery, which will be discussed in details in later sections of this review. Also worth noting is the more recent discovery that AuNP size and shape can modulate the inflammatory responses at the cellular level [104]. In a study that compares gold nanospheres, gold nanorods, and gold nanocubes, Niikura et al. showed that whereas the nanospheres and the nanocubes induced tumor necrosis factor-α (TNF-α), IL-6, IL-12, and granulocyte macrophage colony-stimulating factor (GM-CSF), the nanorods induced interleukin-1β and interleukin-18 via an inflammasome-dependent mechanism [104]. This shape-dependent immunological property may be due to the differing surface energies associated with different nanoscale features, which may promote varying levels of stress upon cellular uptake [108].
Other nanoparticles
Silica nanoparticles offer a range of particle sizes and shapes via controlled synthesis using sol-gel chemistry. An abundance of silanol groups on silica nanoparticle surface allow for functional modifications for increasing specific cellular recognition, facilitating attachment of specific biomolecules, and modulating cellular uptake [109-111]. Nanoscale pores can be integrated into silica nanoparticles, yielding mesoporous silica nanoparticles (MSNs) with more versatile cargo-carrying capacity for vaccination purposes [112, 113]. The pore size and surface functionalization of MSNs can be modified to control the encapsulation and release of antigens or adjuvants of choice [114-117]. Use of silica nanoparticles in vaccine applications include formulations against snake venom, E. coli [118], porcine circovirus [119], HIV [120], and other model antigens [121].
Carbon-based nanoparticles, such as carbon nanotubes and graphene, have also been studied as vaccine carriers [122, 123]. Owing to their high aspect ratio and large surface area, these carbon-based nanoparticles may carry a high proportion of antigens for immune activation. In a study on anticancer vaccination, Villa et al. conjugated single-wall carbon nanotubes (SWCTs) with Wilm's tumour protein, an antigen upregulated in many cancers. Antigen conjugated SWCTs showed good uptake by dendritic cells and macrophages in vitro, and subcutaneous immunization with these SWCTs promoted induction of antigen-specific IgG. In contrast, the free peptide formulation failed to induce an antibody response against the tumor antigen [124]. In another study, graphene nanosheets were used to deliver antigens to facilitate antigen cross-presentation to CD8+ T cells [123]. Xu et al. also demonstrated the use of a dual polymer-modified graphene formulation as an effective adjuvant to enhance the immunogenicity of H. pylori derived antigen (Alum-Ure B) [125].
Semiconductor quantum dots have been applied for vaccine applications, offering a versatile platform for nanoparticulates antigen delivery with the added benefit of particle tracking. In a study by Cambi et al., antigen-conjugated quantum dots of virus-like dimension were shown to combine antigen delivery and bioimaging functionalities, enabling immune cell tracking following antigen uptake. It presented the possibility of tracking antigen-presenting cells in vivo [126]. Along similar lines, Sen et al. showed that quantum dots can induce T cell proliferation and IFN-γ production in vivo while being traceable within lymph nodes [127]. Other nanoparticle platforms with imaging functionality, including polymeric upconversion nanoparticles [128], and iron oxide nanoparticles [129], have also been studied as antigen carriers for vaccine applications. These platforms offer the capability for examining the function mechanisms behind the benefits of nanoparticle vaccines.
Nanoscale morphology and lymph node delivery
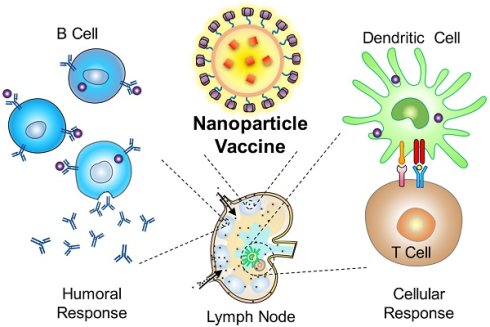
One of the biggest advantages of nanoparticle vaccines is their ability to efficiently drain and accumulate to lymph nodes for enhanced immune processing. The lymphatic system is a subset of the circulatory system that consists of a complex network of vessels, tissues and organs. The lymphatic vessels conduct lymph between different parts of the body. As blood exits the blood vasculature to become interstitial fluid, the lymphatic system provides a return route for this interstitial fluid to the blood vessels in the form of lymph. In the process, it regulates fluid balance within tissues [130]. Underlying the lymphatic system are numerous lymph nodes that scatter throughout the body. These lymph nodes are the homing sites of migratory dendritic cells that present engulfed antigens. Resident in these peripheral lymphoid organs are an abundance of specialized macrophages and other lymphocytes which play a major role in antigen capture and processing for adaptive immune responses. As lymph passes through lymph nodes, resident macrophages further capture passing antigens [131]. In other words, the lymphatic system functions as a filter system of bodily fluids, trapping antigens in the lymph nodes for immune processing.
Viruses and virus-like nanoparticulates can accumulate in lymph nodes via both cell-mediated lymphatic delivery and convective lymphatic transport. The cell-mediated transport is mediated primarily by migratory dendritic cells, which take up antigens outside of the lymphatic system (i.e. skin and lung) and enter lymph nodes via either high endothelial venules or lymph vessels [132, 133]. In comparison to small protein antigens, viruses and nanoparticle vaccines are more favorable to this hitchhiking mechanism. Their particulate nature promotes receptor-mediated, complement-mediated, or other intracellular uptake mechanisms [134, 135]. Antigens associated with nanocarriers are routinely observed to be more efficiently uptaken by dendritic cells compared to soluble antigens, thereby enabling more effective lymph node delivery and cross presentation [128, 136-139]. On the other hand, the nanoscale morphology of viruses and virus-like particulates allows them to move freely in lymphatic vessels to draining lymph nodes. Upon lymph node entry, a special subset of macrophages is responsible for the capture of these nanoparticulates. In a study on lymphatic tracking of viruses by Junt et al., macrophages in the subcapsular sinus and in the medulla of lymph nodes were shown to be responsible for the lymph node accumulation of subcutaneously administered inactivated vesicular stomatitis virus, adenovirus, and vaccinia virus [140]. Depletion of these macrophages resulted in an enhanced virus level that circulated back to the blood, highlighting both viruses' convective transport in the lymphatic system and the macrophages' role in virus filtration. This gatekeeper function by the lymph node-resident macrophages serves to limit blood-borne infection and promote immune processing. Exploiting the same transport mechanisms aimed at detaining viruses, nanoparticle vaccines can effectively target immune cells in lymph nodes, delivering antigens or adjuvants following administration in peripheral tissues [13, 141, 142] (Fig. 3A). The benefit and focus on lymph node targeting also explain why particulate vaccines are most commonly administered subcutaneously as opposed to the conventional intramuscular route for subunit vaccines; free lymphatic drainage and access to immune cells in lymph nodes following injection into the interstitium likely outweigh the “depot effect” afforded by the intramuscular route [143, 144]. Lymphatic targeting by nanoparticles have also been observed following administration via different delivery routes, including pulmonary [145], oral [146], intra-peritoneal [147] routes. Such favorable distribution profile allows tailoring of nanoparticle vaccines towards targeting specific immunological compartments against different infectious threats.
Nanoparticle size and lymph node targeting
Studies on the influence of nanoparticle size on lymph node targeting began in the 1980s as scientists aimed to maximize lymphatic delivery of drugs for treating metastatic cancer. It was generally observed that following subcutaneous injections liposomes smaller than 150 nm were able to enter the lymphatic capillaries whereas larger liposomes remained at the injection sites [148-150]. In a study by Oussoren et al. using isotope labelled liposomes between 40 to 400 nm in diameter in lymphatic tracking, lymphatic uptake was found to be inversely proportional to the liposome size. 40 nm, 70 nm, 170 nm and 400 nm liposomes had approximately 76%, 61%, 30%, and 18% of the injected dose entering the lymphatic system, respectively. Curiously, despite higher lymphatic entry by smaller liposomes in the study, liposome accumulation in the draining lymph node was similar across the differently sized formulations. It was found that the majority of the small liposomes, which consisted of egg-phosphatidylcholine and egg-phosphatidylglycerol, passed through the lymph node and were ultimately captured by the liver and the spleen [151]. The result highlighted the dynamic relationship between particle size and lymph node accumulation; as smaller particles are more likely to enter the lymphatic system, they are also more likely to evade the filtering mechanism of lymph nodes. The authors showed that incorporating phosphotidylserine, a lipid more susceptible to macrophage recognition and capture, increased the lymph node accumulation by 3-fold. Other lipid modification strategies, such as steric stabilization and ligand functionalization, have also been reported to influence the lymph node accumulation of liposomes following lymphatic uptake [152].
(A) Schematics illustrating the mechanisms behind and advantages of lymph node delivery by nanoparticle vaccines. Nanoparticles can exploit both cell-mediated and convective transport for lymph node localization. Particles that enter lymph nodes via interstitial-lymphatic drainage are captured by lymph node-resident macrophages. Enhanced antigen delivery by nanoparticles facilitates antigen presentation and T cell activation. (B) Fluorescence microlymphangiography imaging of 100 nm and 25 nm nanoparticles following tail based injection. 25 nm particles more effectively traverse through the lymphatic network. (C) 25 nm nanoparticles can more efficiently accumulate in lymph nodes as compared to 100 nm nanoparticles as evidenced by fluorescence microscopy. Images in (B)(C) are reproduced with permission from ref. 14, 2007 NPG.
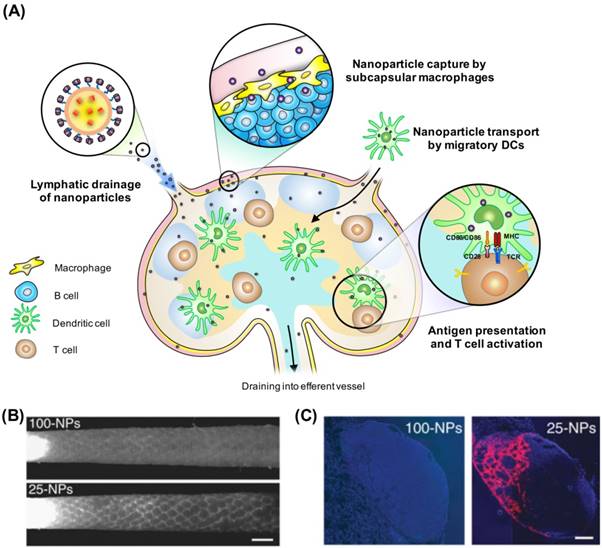
Later studies using solid nanoparticles on examining the effect of particle size on lymph node delivery echo earlier findings based on liposomes. Using polystyrene beads, Manolova et al. confirmed the size-dependent particle transport in the lymphatic system. Upon subcutaneous delivery, large particles between 500 to 2000 nm were found to be associated with dendritic cells from the site of injection, and small nanoparticles between 20 to 200 nm could drain freely in the lymphatic system, effectively targeting lymph node-resident dendritic cells and macrophages [139]. In a study by Reddy et al. on examining nanocarriers as a vaccine delivery platform, Pluronic-stabilized polypropylene sulfide nanoparticles of well-defined sizes were investigated. Using fluorescence microlymphangiography, the investigators showed a clear distinction between 25 nm and 100 nm particles regarding their lymphatic uptake. Following injection into mouse tails, 25 nm particles were efficiently drained to the lymphatic vessels, whereas the interstitial transport of 100 nm particles was less efficient. In contrast to prior studies with liposomes, the 25 nm particles also showed higher lymph node accumulation, resulting in a 10-fold enhancement in lymph node delivery as compared to the 100 nm particles (Fig. 3B, C) [13]. Such enhanced lymph node delivery, which was absent in earlier liposomal studies, could be attributed to both increased colloidal stability of the polymeric nanoparticles and the particles' ability to elicit complement activation. As the polypropylene sulfide particles were functionalized with surface hydroxyl groups to trigger the proteolytic cleavage of C3 complement protein, the danger signal associated with the complement activation could facilitate macrophage uptake upon lymph node entry [153], thereby reducing particle escape from the lymph node's filtering mechanism. The size-dependent lymph node targeting was also observed in other solid particle platforms. In a study by Gao et al. that compared 30 nm and 90 nm gold nanoparticles as antigen carriers, 30 nm gold nanoparticles yielded 2.5-fold higher lymph node accumulation in terms of total gold delivery. Upon conversion to particle number and total particle surface area, 30 nm gold nanoparticles had an enhancement of 67-fold and 7.5-fold as compared to 90 nm particles, respectively [154]. In general, nanoparticles between 20 and 200 nm, a length scale that coincides with viral particles, can exploit interstitial flow for lymphatic delivery. Within this length scale, smaller particle size tends to favor lymph node accumulation.
Antigen delivery by nanoparticle vaccines
Given the privilege of nanocarriers in lymphatic transport, nanoparticles have been shown to enhance the delivery of target antigens to lymph nodes and resident immune cells for processing and immune activation. In the aforementioned study on polypropylene sulfide particles, for instance, Reddy et al. demonstrated increased resident dendritic cell activation in the lymph node by ovalbumin-conjugated nanoparticles. In their animal study, strong anti-ovalbumin humoral response was observed [13], highlighting the benefit of nanoparticle-mediated lymph node delivery on enhancing antigen processing. Moon et al. also showed nanoparticle vaccines can promote preferential accumulation of antigens in the draining lymph nodes and enhance expansion of antigen-specific T cells. Using interbilayer-crosslinked multilamellar vesicles (ICMVs), a lipid-based nanoformulation consisting of multiple layers of lipid vesicles interconnected via thiol chemistry, the investigators demonstrated enhanced antigen delivery to total DCs, macrophages and plasmacytoid DCs in the lymph nodes [50]. Interestingly, liposomes of comparable sizes were much less effective in shuttling antigens to draining lymph nodes in the same study. This observation highlighted the importance of nanoparticle stability in vaccine design as the ICMVs were more colloidally stable than liposomes. ICMV-mediated antigen delivery resulted in significantly higher humoral and cellular responses as compared to the free antigens and the liposomal formulations. The effect of nanoparticle carrier on antigen transport was also shown in a study by Chen et al., who demonstrated effective vaccination against coronaviruses using gold nanoparticle-adsorbed viral antigens. These antigen-coated nanoparticles were structurally analogous to coronaviruses in terms of size and antigen display. Immunofluorescence quantification showed that viral spike proteins delivered with 100 nm gold nanoparticles increased lymph node delivery by approximately 6-fold compared to free spike proteins. These virus-like particles showed high immunogenicity in both murine and avian models and enhanced anti-viral IgA and IgG titers and cellular immune responses in comparison to free protein antigens and a commercial WIV vaccine [155].
Adjuvant delivery by nanoparticle vaccines
In addition to delivering antigens for more effective immune processing, nanoparticles have been extensively applied to localize immunological adjuvants to lymph nodes for improved safety and potency. While conventional adjuvants such as alum have been widely employed clinically to promote humoral responses [156], more recent development in adjuvant research has identified many pathogen-associated molecular patterns (PAMPs) as promising adjuvant candidates towards promoting both humoral and cellular responses [157]. These molecular danger signals are often similar to viral pathogens regarding their immune potentiating mechanisms, triggering innate immunity and in turn facilitating adaptive immune responses. Many PAMPs (i.e. CpG-ODN, Poly(I:C), and cyclic dinucleotides) as well as other molecular agonists of toll-like receptors (TLRs) (i.e. imiquimod and resiquimod) are known to induce strong immune responses. However, their potency poses safety concerns over the likely induction of systemic inflammation. Nanoparticle-based delivery thus offers a desirable strategy in guiding these immunological modulators to lymph nodes, increasing their effective concentration and reducing their systemic reactogenicity. In one example, Nunh et al., constructed a pH-degradable nanogel platform ligated with imidazoquinoline (IMDQ), a TLR7/8 agonist, and showed retention of the adjuvant at the injection site and the draining lymph node. The adjuvant in its free form elicited systemic inflammatory responses, but this side effect was largely obviated with the nanogel formulation. The targeting effect of the nanoformulations also resulted in recruitment of monocytes to the draining lymph node. A large number of immune cells, including B cells, DCs, and macrophages were shown to readily take up these adjuvant-loaded nanogels [158]. In another study by Ilyinskii et. al., synthetic vaccine particles encapsulating resiquimod (R848, a TLR7/8 and TLR9 ligand) augmented humoral and cellular immune responses to both soluble and nanoparticle-delivered proteins compared to that observed with free adjuvants. The adjuvant-loaded nanoparticles promoted local cytokine induction in the lymph nodes and reduced systemic cytokine production observed with free R848. Moreover, while injection of the nanoformulation led to sustained expressions of IFN-γ, IL-12, and IL-1β in lymph nodes after 48 hours, free R848 induced only modest levels of IL-12 and IFN-β [159]. CpG-ODN, an agonist of TLR-9, is another adjuvant that's frequently coupled with nanocarriers for vaccination studies [11, 67, 100, 101, 160, 161]. Some of the primary advantages of nanoparticle-based CpG formulations include strong T cell responses, dosage sparing, and reduced systemic side effects. Such formulations have been commonly applied in anticancer vaccination efforts owing to the need for high cell-based immune responses for effective tumor containment.
Co-delivery of antigens and adjuvants by nanoparticle vaccines
Transport of viral antigen and immune-potentiating adjuvants by viruses to immune cells is a highly coordinated event as viral particles shuttle both antigen targets and adjuvanting nucleic acids simultaneously. Such antigen/adjuvant coordination, or its absence, has been shown to strongly influence immune cell activation [162]. In addition, the immune system can respond to viruses through multiple PAMPs, including glycoprotein, DNA, dsRNA, and ssRNA, activating a broad spectrum of signalling pathways for heightened antiviral immunity [163]. The synthetic flexibility of nanoparticles has thus been exploited to emulate the co-delivery capacity of viral pathogens to boost immune responses. In some cases, antigen and adjuvant are localized on the same nanoparticles for synchronized delivery. Ma et al. incorporated a hepatitis B surface antigen (HBsAg) and CpG adjuvant onto PLGA nanoparticles via conjugation with dopamine, allowing the particles to display both the viral antigen and the immune activator. The study showed that the pathogen-mimicking particle enhanced the recruitment of immune cells to the injected site, activated bone marrow derived dendritic cells, and induced strong humoral and cellular immune responses [164]. In another study conducted by Kuai et al., a disc-like synthetic high-density lipoprotein (sHDL) was applied to integrate both target antigens of CD8+ T cells and CpG-ODN for anticancer vaccination. The nanodisc drastically improved the co-delivery of antigens and adjuvants to lymph nodes compared to soluble vaccine and induced a 31-fold enhancement in antigen-specific CD8+ cytotoxic T-lymphocyte response as compared to the free peptide and adjuvant control.
Besides incorporating antigens and adjuvants on the same particle for co-delivery, a number of studies demonstrated that delivering antigen and adjuvant in separate but similar nanocarriers can also elevate antigen-specific immune responses. In one example, an HIV antigen (HIVgp41) was anchored on the surface of liposomes and co-delivered with a liposomal formulation of cd-GMP, a potent agonist of the STING pathway. The study showed a substantial accumulation of the STING agonist in draining lymph nodes [165], and it is expected that the liposome-bound peptide antigen was delivered in a similar fashion. As a result, enhanced activation of antigen presenting cells and increased levels of antibodies against the HIV antigen were observed. Immune stimulation by formulations containing separate antigen- and adjuvant-loaded nanoparticles was also reported in a study exploring the benefit of multi-adjuvant loaded particles. By incorporating multiple distinctive activators of TLRs, including monophosphoryl lipid A (a TLR4 agonist) and R837 (a TLR7 agonist), Kasturi et al. demonstrated adjuvant synergism that triggered elevated antigen-specific humoral responses [70]. Using ovalbumin and hemagglutinin of influenza viruses, the investigators demonstrated long-lasting humoral responses and evidence of memory B cell formation following immunization with the nanoparticle vaccine. These examples highlight the functional versatility of synthetic nanoparticles, which can facilitate different modes of virus mimicry for immune activation.
Repetitive antigen display for immune activation
In addition to the role of lymph nodes in trapping nanoscale particles for immune processing, the immune system has also adapted to the repetitive antigen display on viruses for effective potentiation. Virus surfaces display antigenic epitopes in an ordered and highly repetitive fashion, and the presentation of repetitively arranged and appropriately spaced antigens on the surface of virus or virus-like particles has been linked to enhanced immune responses [166]. Many reports have shown that numerous components of the mammalian immune system have evolved to respond strongly to the repetitive antigen patterns frequently found on pathogens [167-169]. In comparison, non-repetitive antigens are usually less effective in inducing immune responses [170-173]. Repetitive motifs on viral surfaces are also found to activate the complement system [174] engaging the CD19-CD21 complex, which further facilitates B cell activation and amplify other immune processing pathways [175].
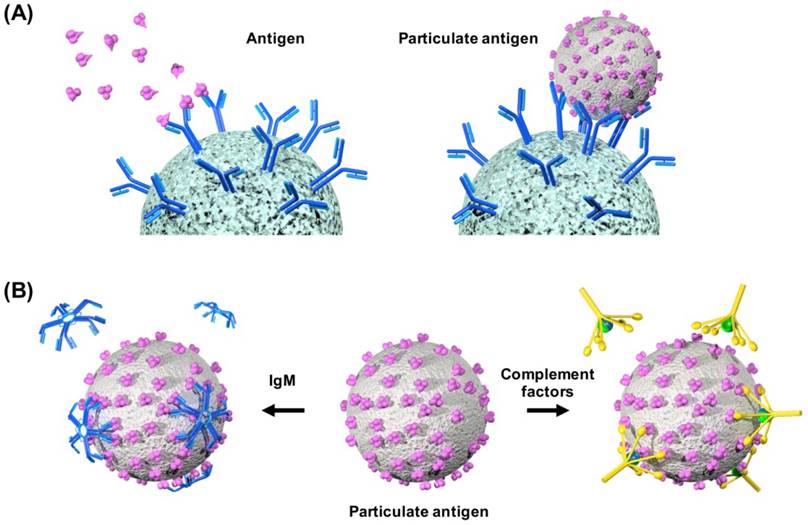
Understanding the link between structural features of antigen display and immunological induction is vital in designing nanoparticulate vaccines. Multivalent interactions promote B-cell receptor (BCR) clustering and signaling and facilitate receptor-mediated internalization of antigen. Antigen features, such as epitope affinity, valency, or co-receptor recruitment can impact B and T cell signaling. In a study that used antigen-conjugated polymer to assay the impact of antigen valency on B cell activation, Puffer et al. showed that clustering of BCRs by multivalent antigens is crucial for antigen-dependent signaling. The antigen-conjugated polymers clustered unbound BCRs and contributed to enhanced intracellular signalling [176]. Whereas the multivalent antigen-polymer conjugates elicited antibody production, free antigens failed to trigger humoral responses (Fig. 4A). Highly repetitive surfaces are also known to bind strongly to natural IgM antibodies through multivalent, high-avidity interactions [177]. Such antibody binding also facilitates cellular uptake of particles by macrophages and dendritic cells, which can in turn enhance immune processing through increased antigen presentation. It is also worth noting that many important components in the humoral arm of innate immunity, such as complement C1q, pentraxins, ficolins and collectins, are multimeric structures that favor high-avidity interactions with repetitive pathogen surfaces [178] (Fig. 4B). These observations highlight how the immune system has been primed to respond to repetitive motifs frequently found on viral particles.
Unlike free subunit antigens, nanoparticle vaccines present a high concentration of antigens on their surfaces. A nanoparticle can be surface functionalized with up to hundreds of antigens, effectively emulating the antigen display on viral surfaces [179, 180]. The multivalent antigen display on nanoparticles enhances antibody responses by efficiently cross-linking BCRs, activating complement, and facilitating antibody binding. These factors play synergistic roles in promoting B cell differentiation and stimulating DC-mediated T cell priming. Although synthetically prepared nanoparticles have yet to show the level of ordered antigen arrangement found on viruses and virus-like particles derived from cell culture systems [181, 182], ongoing studies continue to demonstrate emerging techniques to couple antigens of interest to nanocarriers. In the last section of the review, we review commonly used methods for coupling antigens with nanoparticles. These strategies may spur novel approaches for preparing nanoparticle vaccines.
Strategies for antigen functionalization in nanoparticle vaccines
Association of protein antigens with nanoparticles can be divided into particle encapsulation and surface association. While many studies show excellent nanoparticle vaccine potency with encapsulated antigens, surface associated antigen may benefit from the innate immune factors and immune processing mechanisms described in the previous section. Techniques to associate protein or peptide antigens can be categorized into multiple categories. The different modes of antigen association are highlighted as follows.
(A) Schematics showing multivalent interactions by nanoparticle vaccines promote B cell receptor clustering and facilitate receptor-mediated internalization. (B) Schematics illustrating enhanced binding of multimeric immune factors, such as IgM and complement factors, to nanoparticle vaccines.
Chemical conjugation
Thiol and amine groups on protein or peptide antigens are frequently exploited for bioconjugation with nanoparticles. The thiol group on a cysteine amino acid is a powerful nucleophile with the capacity to form covalent linkage. Several linker groups, such as maleimide and succinimidyl 3-(2-pyridyldithio)propionate (SPDP), facilitate the conjugation between thiol-containing antigens with nanoparticles [183]. In a maleimide-thiol reaction, the nucleophilic thiolate anion attacks the π-bond of maleimide, forming the enolate intermediate and yielding the desired conjugate. This technique has been widely used to assemble nanoparticle vaccines [184-186]. In a study on refining a liposomal formulation of HIV vaccine, thiol chemistry was exploited to control the physiological conformation and density of the target antigen to modulate immune responses [186]. Thiols groups also readily associate with gold surfaces. The strong interaction between sulfur and gold drives the sulfur atom to fill the free orbitals of a gold atom, creating a coordinate covalent bond [187, 188]. Such approach has been extensively applied to associate nucleic acids [188-192] and antigens [193, 194] with gold nanoparticles.
Amine groups, on the other hand, are present on all protein and peptide antigens, which can be linked to nanocarriers via amide bond formation, typically through carbodiimide crosslinker chemistry. Bioconjugations with amine-containing antigens are commonly performed using 1-ethyl-3-(3-(dimethylamino)propyl)carbodi-imide (EDC) and N-hydroxysuccinimide (NHS). NHS or its sulfonated from (sulfo-NHS) is efficiently coupled to carboxyl groups with the aid of EDC to form NHS esters. The NHS esters then covalently conjugate to primary amines to form an intermediate compound that is subsequently hydrolysed to the desired conjugate [195]. Many nanoparticle vaccines have been prepared with the EDC/NHS conjugation method with a high coupling yield [196, 197]. Polydopamine functionalization is another conjugation approach that is increasingly applied to associate proteins and peptides with nanoparticles. In alkaline pH, dopamine undergoes oxidative self-polymerization to form a layer of polydopamine that can coat almost any type of material [198-200]. This mussel-like adhesive layer enables a secondary reaction with biomolecules containing thiol or amine groups [201, 202]. The technique has been demonstrated on both organic and inorganic nanoparticles [203, 204]. Dopamine-incorporated polymers have also been employed to adsorb proteins for macrophage-targeted delivery [205]. The versatile technique can be applied to conjugate multiple cargoes for vaccine applications [164].
Electrostatic interaction
Electrostatic attraction between oppositely charged antigens and nanoparticles have been exploited to prepare nanoparticle vaccines. In general, cationic nanocarriers are prepared for the association of anionic protein antigens. In the case of liposomes, cationic lipid DOTAP is frequently applied to render the nanocarrier positively charged. DOTAP-based liposomes have been shown to absorb HPV E7 peptides [206], enhancing antigen-specific CD8 T cell response and increasing antitumor responses. Strategies have also been employed to prepare antigens with added anionic moieties via recombinant protein engineering. By expressing HPV E7 protein and ovalbumin with an anionic lipoprotein, Shen et al. demonstrated enhanced antigen association efficiency and retention to DOTAP liposomes. The vaccine formulation elevated antigen-specific cellular responses and inhibited tumor growth in a mouse model [207, 208].
In another work based on PRINT (Particle Replication in Non-wetting Templates) technology, electrostatic interaction was exploited to generate nanoparticle vaccines of cylindrical shape. Cationic particles were prepared by blending positively charged polymers with PLGA prior to the imprint lithography process. Upon mixing with anionic hemagglutinin proteins of influenza viruses, a high level of antigen binding to the particle surface was achieved [209]. Immunization with the nanoparticle vaccine elicited a more potent anti-influenza antibody response compared to a commercial vaccine based on inactivated subunit influenza viruses. Unlike other spherical nanoparticle vaccines, the platform offers the ability to mimic the filamentous shape that can be found among many virus species.
Physical adsorption
Synthetic nanoparticles have high surface energies owing to their large radii of curvature. As a result, adsorption of protein can occur spontaneously owing to a combination of weak interaction forces, leading to the formation of protein corona formation [108]. This phenomenon was demonstrated to facilitate the assembly of synthetic virus-like nanoparticles [155]. Upon mixing gold nanoparticles with viral antigens, rapid protein adsorption took place on the nanoparticle surface. This adsorption led to colloidal stabilization of gold nanoparticles, preventing their aggregation in biological buffers. Biochemical analysis showed the facile assembly method surrounded each nanoparticle with approximately 900 coronavirus spike proteins, inducing enhanced anti-viral immune responses. The adsorption approach has been used extensively to associated antigens with nanocarriers, including nanoparticles made of gold [210], calcium phosphate [211, 212], aluminium hydroxide [213], carbon [214, 215], silica [117, 216], and organic polymers [217, 218]. The range of adsorbed biomolecules vary from DNA [217, 219, 220], adjuvant [221], small peptides, and protein antigens [155, 218, 222, 223]. It is important to note that formation of protein corona is a dynamic process that can be strongly influenced by both nanomaterials and antigen of interest [224]. Studies have also shown that antigens can undergo conformational changes upon nanoparticle adsorption [225], which could have both positive and negative effects on the resulting vaccine formulations.
Biomembrane coating
Coating nanoparticles with cellular membranes is an emerging functionalization approach that can pave ways to novel vaccine formulations with virus-mimetic features. It has been shown that following dispersion of cell membrane vesicles with nanoparticles, highly controlled membrane coating can be achieved, yielding unilamellar membrane cloaked nanoparticles retaining membrane proteins in their right-side-out orientation [226-228]. These nanoparticles are structurally analogous to enveloped viruses consisting of cell-derived lipid membranes stabilized via viral capsids or matrix proteins [229]. The membrane coating approach has been adapted for vaccine preparations against cancer and bacteria [154, 230-232]. Enhanced dendritic cell activation and presentation of a melanoma-associated tumor antigen (gp100) was demonstrated by Fang et al. using PLGA nanoparticles cloaked in B16-F10 membranes [232]. The enhanced immune response to the membrane antigen was attributed to increased cellular delivery and colocalization with immunological adjuvant facilitated by the nanoparticles. Gao et al. also showed that bacterial outer membrane vesicles (OMVs) can be rendered more immunogenic following coating on gold nanoparticles. The OMV-coated nanoparticles significantly increased antigen delivery to lymph nodes and elevated production of cytokines associated with bacterial containment [233]. The membrane cloaking approach offers a titillating strategy towards future vaccine designs as the method enables coupling of membrane-anchored antigens in their native conformation with immunogenic nanocarriers.
Concluding remarks
Advances in nanotechnology and its adoption in vaccinology have helped push the boundaries of non-live, subunit vaccines, resulting in many exciting demonstrations of effective immune potentiation by nanoformulations. Not only can nanoparticle vaccines enhance humoral responses against target antigens, they have been shown to promote cell-based immunity as well as immunological memory. These are hallmarks of good vaccine formulations that are often inherent to live attenuated viruses. An increasing number of studies have shed light on the mechanisms behind the benefits of nanoparticle vaccines, including lymph node targeting, multivalent antigen display, and coordinated delivery of antigens and adjuvants. These features can find their mechanistic analogues in the immunological processing of viruses. Given the frequent semblance between nanoparticle vaccines and viruses regarding their size, morphology, antigen display, and adjuvanticity effect, nanoparticles present a compelling platform in bridging the gap between live and non-live vaccines. Emerging techniques in nanoparticle functionalization also pave ways to novel formulation designs that promise controlled immune modulation. Going forward, understanding of virology may assist scientists and engineering in preparing emerging nanoformulations with advanced virus-like features. Such vaccine nanotechnology is envisioned to improve vaccine safety, potency, and availability, offering compelling platforms towards addressing the many public health threats yet to have effective prophylaxis and treatment options.
Acknowledgements
The authors acknowledge support from the Ministry of Science and Technology, R.O.C. (105-2119-M-001-014 and 106-2321-B-001-034).
Competing Interests
The authors have declared that no competing interest exists.
References
1. F. Fenner DAH, I. Arita, Z. JeZek, I. D. Ladnyi. Smallpox and its Eradication. World Health Organization Geneva. 1988
2. Pulendran B, Ahmed R. Translating innate immunity into immunological memory: Implications for vaccine development. Cell. 2006;124:849-63
3. Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C. et al. Nanoparticle vaccines. Vaccine. 2014;32:327-37
4. Sahdev P, Ochyl LJ, Moon JJ. Biomaterials for nanoparticle vaccine delivery systems. Pharmaceutical research. 2014;31:2563-82
5. Chahal JS, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD. et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. P Natl Acad Sci USA. 2016;113:E4133-E42
6. Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH. et al. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. The Journal of clinical investigation. 2015;125:2532-46
7. Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand T-fh cells and promote germinal center induction. P Natl Acad Sci USA. 2012;109:1080-5
8. Chahal JS, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD. et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proceedings of the National Academy of Sciences. 2016;113:E4133-E42
9. Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2016;16:489-496
10. Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396-401
11. Jeanbart L, Ballester M, de Titta A, Corthesy P, Romero P, Hubbell JA. et al. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol Res. 2014;2:436-47
12. Li AV, Moon JJ, Abraham W, Suh H, Elkhader J, Seidman MA. et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Science translational medicine. 2013;5:204ra130
13. Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature biotechnology. 2007;25:1159-64
14. Henriksen-Lacey M, Korsholm KS, Andersen P, Perrie Y, Christensen D. Liposomal vaccine delivery systems. Expert Opin Drug Deliv. 2011;8:505-19
15. Heurtault B, Frisch B, Pons F. Liposomes as delivery systems for nasal vaccination: strategies and outcomes. Expert Opin Drug Deliv. 2010;7:829-44
16. Gregoriadis G. TARGETING OF DRUGS: IMPLICATIONS IN MEDICINE. The Lancet. 1981;318:241-7
17. Ryman BE, Tyrrell DA. Liposomes-bags of potential. Essays in biochemistry. 1980;16:49-98
18. Yatvin MB, Lelkes PI. Clinical prospects for liposomes. Medical physics. 1982;9:149-75
19. Muro S, Koval M, Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Current vascular pharmacology. 2004;2:281-99
20. Hart SL. Lipid carriers for gene therapy. Current drug delivery. 2005;2:423-8
21. Allison AG, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252:252
22. Gregoriadis G. Immunological adjuvants: a role for liposomes. Immunology today. 1990;11:89-97
23. Takagi A, Matsui M, Ohno S, Duan H, Moriya O, Kobayashi N. et al. Highly efficient antiviral CD8+ T-cell induction by peptides coupled to the surfaces of liposomes. Clinical and vaccine immunology: CVI. 2009;16:1383-92
24. Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E. et al. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int J Pharm. 2010;392:218-23
25. Shahum E, Thérien H-M. Effect of liposomal antigens on the priming and activation of the immune system by dendritic cells. Int Immunopharmacol. 2002;2:591-601
26. Peine KJ, Gupta G, Brackman DJ, Papenfuss TL, Ainslie KM, Satoskar AR. et al. Liposomal resiquimod for the treatment of Leishmania donovani infection. J Antimicrob Chemoth. 2014;69:168-75
27. Barnier-Quer C, Elsharkawy A, Romeijn S, Kros A, Jiskoot W. Adjuvant effect of cationic liposomes for subunit influenza vaccine: influence of antigen loading method, cholesterol and immune modulators. Pharmaceutics. 2013;5:392-410
28. Matyas GR, Mayorov AV, Rice KC, Jacobson AE, Cheng KJ, Iyer MR. et al. Liposomes containing monophosphoryl lipid A: A potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine. 2013;31:2804-10
29. Peachman KK, Jobe O, Wieczorek L, Asher L, Polonis VR, Rao V. et al. Liposomes Containing Glucosyl Ceramide and the Adjuvant Monophosphoryl Lipid A Specifically Bind T4 Bacteriophage: A Self-Assembling Nanocarrier. Aids Res Hum Retrov. 2011;27:A37-A
30. Yamamoto Y, Sahara H, Takenouchi M, Matsumoto Y, Imai A, Fujita T. et al. Inhibition of CD62L(+) T-cell response in vitro via a novel sulfo-glycolipid, beta-SQAG9 liposome that binds to CD62L molecule on the cell surface. Cellular immunology. 2004;232:105-15
31. Ohtsuro Y, Furukawa M, Imagawa T, Sugimoto N, Ikutoh M, Nakatsugi S. et al. Growth-Inhibition of Tumor-Cells by a Liposome-Encapsulated, Mycolic Acid-Containing Glycolipid, Trehalose 2,3,6'-Trimycolate. Immunology. 1991;74:497-503
32. Badiee A, Jaafari MR, Samiei A, Soroush D, Khamesipour A. Coencapsulation of CpG oligodeoxynucleotides with recombinant Leishmania major stress-inducible protein 1 in liposome enhances immune response and protection against leishmaniasis in immunized BALB/c mice. Clinical and Vaccine Immunology. 2008;15:668-74
33. Kim D, Kwon S, Ahn CS, Lee Y, Choi SY, Park J. et al. Adjuvant effect of liposome-encapsulated natural phosphodiester CpG-DNA. Bmb Rep. 2011;44:758-63
34. Kim D, Rhee JW, Kwon S, Kim YE, Choi SY, Park J. et al. Enhancement of immunomodulatory activity by liposome-encapsulated natural phosphodiester bond CpG-DNA in a human B cell line. Bmb Rep. 2010;43:250-6
35. Kwon S, Kim D, Park BK, Wu G, Park MC, Ha YW. et al. Induction of immunological memory response by vaccination with TM4SF5 epitope-CpG-DNA-liposome complex in a mouse hepatocellular carcinoma model. Oncol Rep. 2013;29:735-40
36. Miyabe H, Hyodo M, Nakamura T, Sato Y, Hayakawa Y, Harashima H. A new adjuvant delivery system 'cyclic di-GMP/YSK05 liposome' for cancer immunotherapy. Journal of controlled release: official journal of the Controlled Release Society. 2014;184:20-7
37. Huckriede A, Bungener L, Stegmann T, Daemen T, Medema J, Palache AM. et al. The virosome concept for influenza vaccines. Vaccine. 2005;23:S26-S38
38. Kaneda Y. Virosomes: evolution of the liposome as a targeted drug delivery system. Advanced drug delivery reviews. 2000;43:197-205
39. Arkema A, Huckriede A, Schoen P, Wilschut J, Daemen T. Induction of cytotoxic T lymphocyte activity by fusion-active peptide-containing virosomes. Vaccine. 2000;18:1327-33
40. Bovier PA. Epaxal®: a virosomal vaccine to prevent hepatitis A infection. Expert review of vaccines. 2008;7:1141-50
41. Moser C, Amacker M, Kammer AR, Rasi S, Westerfeld N, Zurbriggen R. Influenza virosomes as a combined vaccine carrier and adjuvant system for prophylactic and therapeutic immunizations. Expert review of vaccines. 2007;6:711-21
42. Moser C, Müller M, Kaeser MD, Weydemann U, Amacker M. Influenza virosomes as vaccine adjuvant and carrier system. Expert review of vaccines. 2013;12:779-91
43. Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787-96
44. Bungener L, Serre K, Bijl L, Leserman L, Wilschut J, Daemen T. et al. Virosome-mediated delivery of protein antigens to dendritic cells. Vaccine. 2002;20:2287-95
45. Angel J, Chaperot L, Molens J-P, Mezin P, Amacker M, Zurbriggen R. et al. Virosome-mediated delivery of tumor antigen to plasmacytoid dendritic cells. Vaccine. 2007;25:3913-21
46. Schumacher R, Adamina M, Zurbriggen R, Bolli M, Padovan E, Zajac P. et al. Influenza virosomes enhance class I restricted CTL induction through CD4+ T cell activation. Vaccine. 2004;22:714-23
47. Moser C, Amacker M, Zurbriggen R. Influenza virosomes as a vaccine adjuvant and carrier system. Expert review of vaccines. 2011;10:437-46
48. Kunisawa J, Nakanishi T, Takahashi I, Okudaira A, Tsutsumi Y, Katayama K. et al. Sendai Virus Fusion Protein-Mediates Simultaneous Induction of MHC Class I/II-Dependent Mucosal and Systemic Immune Responses Via the Nasopharyngeal-Associated Lymphoreticular Tissue Immune System. The Journal of Immunology. 2001;167:1406-12
49. Lyles DS, Mckenzie M, Parce JW. Subunit Interactions of Vesicular Stomatitis-Virus Envelope Glycoprotein Stabilized by Binding to Viral Matrix Protein. Journal of virology. 1992;66:349-58
50. Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B. et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243-51
51. Nguyen DN, Mahon KP, Chikh G, Kim P, Chung H, Vicari AP. et al. Lipid-derived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc Natl Acad Sci U S A. 2012;109:E797-803
52. Perche F, Benvegnu T, Berchel M, Lebegue L, Pichon C, Jaffres PA. et al. Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine. 2011;7:445-53
53. Jensen S, Thomsen AR. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. Journal of virology. 2012;86:2900-10
54. Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373-81
55. Li X, Deng X, Yuan M, Xiong C, Huang Z, Zhang Y. et al. In vitro degradation and release profiles of poly-DL-lactide-poly(ethylene glycol) microspheres with entrapped proteins. Journal of Applied Polymer Science. 2000;78:140-8
56. Nochi T, Yuki Y, Takahashi H, Sawada S-i, Mejima M, Kohda T. et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater. 2010;9:572-8
57. K. Garg N, Mangal S, Khambete H, K. Sharma P, K. Tyagi R. Mucosal Delivery of Vaccines: Role of Mucoadhesive/Biodegradable Polymers. Recent Patents on Drug Delivery & Formulation. 2010;4:114-28
58. Csaba N, Garcia-Fuentes M, Alonso MJ. Nanoparticles for nasal vaccination. Advanced Drug Delivery Reviews. 2009;61:140-57
59. Gupta RK, Chang AC, Siber GR. Biodegradable polymer microspheres as vaccine adjuvants and delivery systems. Dev Biol Stand. 1998;92:63-78
60. Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. European Journal of Pharmaceutics and Biopharmaceutics. 2007;65:259-69
61. Lü J-M, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q. et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Review of Molecular Diagnostics. 2009;9:325-41
62. Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Current Opinion in Solid State and Materials Science. 2002;6:319-27
63. Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: An overview of biomedical applications. Journal of Controlled Release. 2012;161:505-22
64. Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci U S A. 2012;109:1080-5
65. Chong CS, Cao M, Wong WW, Fischer KP, Addison WR, Kwon GS. et al. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. Journal of controlled release: official journal of the Controlled Release Society. 2005;102:85-99
66. Manish M, Rahi A, Kaur M, Bhatnagar R, Singh S. A Single-Dose PLGA Encapsulated Protective Antigen Domain 4 Nanoformulation Protects Mice against Bacillus anthracis Spore Challenge. PLOS ONE. 2013;8:e61885
67. Diwan M, Elamanchili P, Cao M, Samuel J. Dose sparing of CpG oligodeoxynucleotide vaccine adjuvants by nanoparticle delivery. Current drug delivery. 2004;1:405-12
68. Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM. et al. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33:4957-64
69. Ilyinskii P, Roy C, O'Neil C, Browning E, Pittet L, Altreuter D. et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine. 2014;19:2882-95
70. Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI. et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543-7
71. Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA. et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348:aaa8205
72. Coumes F, Huang CY, Huang CH, Coudane J, Domurado D, Li S. et al. Design and Development of Immunomodulatory Antigen Delivery Systems Based on Peptide/PEG-PLA Conjugate for Tuning Immunity. Biomacromolecules. 2015;16:3666-73
73. Hasegawa K, Noguchi Y, Koizumi F, Uenaka A, Tanaka M, Shimono M. et al. In vitro stimulation of CD8 and CD4 T cells by dendritic cells loaded with a complex of cholesterol-bearing hydrophobized pullulan and NY-ESO-1 protein: Identification of a new HLA-DR15-binding CD4 T-cell epitope. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:1921-7
74. Uenaka A, Wada H, Isobe M, Saika T, Tsuji K, Sato E. et al. T cell immunomonitoring and tumor responses in patients immunized with a complex of cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein. Cancer Immunity: a Journal of the Academy of Cancer Immunology. 2007;7:9
75. Muraoka D, Harada N, Hayashi T, Tahara Y, Momose F, Sawada S. et al. Nanogel-based immunologically stealth vaccine targets macrophages in the medulla of lymph node and induces potent antitumor immunity. ACS nano. 2014;8:9209-18
76. Li P, Luo Z, Liu P, Gao N, Zhang Y, Pan H. et al. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J Control Release. 2013;168:271-9
77. Borges O, Cordeiro-da-Silva A, Tavares J, Santarém N, de Sousa A, Borchard G. et al. Immune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate coated chitosan nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 2008;69:405-16
78. Feng G, Jiang Q, Xia M, Lu Y, Qiu W, Zhao D. et al. Enhanced Immune Response and Protective Effects of Nano-chitosan-based DNA Vaccine Encoding T Cell Epitopes of Esat-6 and FL against Mycobacterium Tuberculosis Infection. PLOS ONE. 2013;8:e61135
79. Thomann-Harwood LJ, Kaeuper P, Rossi N, Milona P, Herrmann B, McCullough KC. Nanogel vaccines targeting dendritic cells: Contributions of the surface decoration and vaccine cargo on cell targeting and activation. Journal of Controlled Release. 2013;166:95-105
80. Zhao F, Zhang X, Liu S, Zeng T, Yu J, Gu W. et al. Assessment of the immune responses to Treponema pallidum Gpd DNA vaccine adjuvanted with IL-2 and chitosan nanoparticles before and after Treponema pallidum challenge in rabbits. Science China Life Sciences. 2013;56:174-80
81. Zhao K, Chen G, Shi X-m, Gao T-t, Li W, Zhao Y. et al. Preparation and Efficacy of a Live Newcastle Disease Virus Vaccine Encapsulated in Chitosan Nanoparticles. PLOS ONE. 2012;7:e53314
82. Nanda RK, Edao BM, Hajam IA, Sekar SC, Ganesh K, Bhanuprakash V. et al. An effective mannosylated chitosan nanoparticle DNA vaccine for FMD virus. Virologica Sinica. 2012;27:373-6
83. Akagi T, Baba M, Akashi M. Biodegradable Nanoparticles as Vaccine Adjuvants and Delivery Systems: Regulation of Immune Responses by Nanoparticle-Based Vaccine. In: (ed.) Kunugi S, Yamaoka T. Polymers in Nanomedicine. Berlin, Heidelberg: Springer Berlin Heidelberg. 2012:31-64
84. Arca HC, Gunbeyaz M, Senel S. Chitosan-based systems for the delivery of vaccine antigens. Expert review of vaccines. 2009;8:937-53
85. Chua BY, Al Kobaisi M, Zeng W, Mainwaring D, Jackson DC. Chitosan Microparticles and Nanoparticles as Biocompatible Delivery Vehicles for Peptide and Protein-Based Immunocontraceptive Vaccines. Molecular Pharmaceutics. 2012;9:81-90
86. Riteau N, Sher A. Chitosan: An Adjuvant with an Unanticipated STING. Immunity. 2016;44:522-4
87. Civril F, Deimling T, Mann CCD, Ablasser A, Moldt M, Witte G. et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498:332-7
88. Debache K, Kropf C, SchÜTz CA, Harwood LJ, KÄUper P, Monney T. et al. Vaccination of mice with chitosan nanogel-associated recombinant NcPDI against challenge infection with Neospora caninum tachyzoites. Parasite Immunology. 2011;33:81-94
89. Akagi T, Kaneko T, Kida T, Akashi M. Preparation and characterization of biodegradable nanoparticles based on poly(gamma-glutamic acid) with l-phenylalanine as a protein carrier. J Control Release. 2005;108:226-36
90. Kalkanidis M, Pietersz GA, Xiang SD, Mottram PL, Crimeen-Irwin B, Ardipradja K. et al. Methods for nano-particle based vaccine formulation and evaluation of their immunogenicity. Methods. 2006;40:20-9
91. Scheerlinck JP, Gloster S, Gamvrellis A, Mottram PL, Plebanski M. Systemic immune responses in sheep, induced by a novel nano-bead adjuvant. Vaccine. 2006;24:1124-31
92. Arias JL, Gallardo V, Gómez-Lopera SA, Plaza RC, Delgado AV. Synthesis and characterization of poly(ethyl-2-cyanoacrylate) nanoparticles with a magnetic core. Journal of Controlled Release. 2001;77:309-21
93. Fontana G, Pitarresi G, Tomarchio V, Carlisi B, San Biagio PL. Preparation, characterization and in vitro antimicrobial activity of ampicillin-loaded polyethylcyanoacrylate nanoparticles. Biomaterials. 1998;19:1009-17
94. Kreuter J, Speiser PP. New adjuvants on a polymethylmethacrylate base. Infection and Immunity. 1976;13:204-10
95. Kreuter J MR, Gruschkau H, Speiser PP. The use of new polymethylmethacrylate adjuvants for split influenza vaccines. Exp Cell Biol. 1976;44:12-9
96. Patravale V, Prabhu P. Potential of Nanocarriers in Antigen Delivery: The Path to Successful Vaccine Delivery. Nanocarriers. 2014;1:10-45
97. Adam Yuh Lin JL, Adham Sean Bear, Joseph Keith Young, Phillip Eckels, Laureen Luo, Aaron Edward Foster, Rebekah Anna Drezek. High-density sub-100-nm peptide-gold nanoparticle complexes improve vaccine presentation by dendritic cells in vitro. Nanoscale Research Letters. 2013;8:72
98. Fuller DH, Loudon P, Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40:86-97
99. Pokharkar V, Bhumkar D, Suresh K, Shinde Y, Gairola S, Jadhav SS. Gold Nanoparticles as a Potential Carrier for Transmucosal Vaccine Delivery. Journal of biomedical nanotechnology. 2011;7:57-9
100. Lin AY, Almeida JPM, Bear A, Liu N, Luo L, Foster AE. et al. Gold Nanoparticle Delivery of Modified CpG Stimulates Macrophages and Inhibits Tumor Growth for Enhanced Immunotherapy. PloS one. 2013;8:e63550
101. Radovic-Moreno AF, Chernyak N, Mader CC, Nallagatla S, Kang RS, Hao L. et al. Immunomodulatory spherical nucleic acids. Proc Natl Acad Sci U S A. 2015;112:3892-7
102. Xu L, Liu Y, Chen Z, Li W, Liu Y, Wang L. et al. Surface-Engineered Gold Nanorods: Promising DNA Vaccine Adjuvant for HIV-1 Treatment. Nano Letters. 2012;12:2003-12
103. Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60:915-28
104. Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y. et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS nano. 2013;7:3926-38
105. Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol. 2013;3:13
106. John WS, Natalie JT, David LB, Sam JK, David WW, James EC Jr. Gold nanorod vaccine for respiratory syncytial virus. Nanotechnology. 2013;24:295102
107. Dolja VV, Boyko VP, Agranovsky AA, Koonin EV. Phylogeny of capsid proteins of rod-shaped and filamentous RNA plant viruses: two families with distinct patterns of sequence and probably structure conservation. Virology. 1991;184:79-86
108. Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P. et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543-57
109. He X-x, Wang K, Tan W, Liu B, Lin X, He C. et al. Bioconjugated Nanoparticles for DNA Protection from Cleavage. Journal of the American Chemical Society. 2003;125:7168-9
110. Xia T, Kovochich M, Liong M, Meng H, Kabehie S, George S. et al. Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of siRNA and DNA Constructs. ACS Nano. 2009;3:3273-86
111. Yu M, Jambhrunkar S, Thorn P, Chen J, Gu W, Yu C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale. 2013;5:178-83
112. Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710-2
113. Niu Y, Popat A, Yu M, Karmakar S, Gu W, Yu C. Recent advances in the rational design of silica-based nanoparticles for gene therapy. Therapeutic Delivery. 2012;3:1217-37
114. Manzano M, Aina V, Areán CO, Balas F, Cauda V, Colilla M. et al. Studies on MCM-41 mesoporous silica for drug delivery: Effect of particle morphology and amine functionalization. Chemical Engineering Journal. 2008;137:30-7
115. Mody KT, Popat A, Mahony D, Cavallaro AS, Yu C, Mitter N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale. 2013;5:5167-79
116. Carvalho LV, Ruiz RdC, Scaramuzzi K, Marengo EB, Matos JR, Tambourgi DV. et al. Immunological parameters related to the adjuvant effect of the ordered mesoporous silica SBA-15. Vaccine. 2010;28:7829-36
117. Mahony D, Cavallaro AS, Stahr F, Mahony TJ, Qiao SZ, Mitter N. Mesoporous Silica Nanoparticles Act as a Self-Adjuvant for Ovalbumin Model Antigen in Mice. Small. 2013;9:3138-46
118. Mercuri LP, Carvalho LV, Lima FA, Quayle C, Fantini MCA, Tanaka GS. et al. Ordered Mesoporous Silica SBA-15: A New Effective Adjuvant to Induce Antibody Response. Small. 2006;2:254-6
119. Guo HC, Feng XM, Sun SQ, Wei YQ, Sun DH, Liu XT. et al. Immunization of mice by hollow mesoporous silica nanoparticles as carriers of porcine circovirus type 2 ORF2 protein. Virology journal. 2012;9:108
120. Cheng K, El-Boubbou K, Landry CC. Binding of HIV-1 gp120 glycoprotein to silica nanoparticles modified with CD4 glycoprotein and CD4 peptide fragments. ACS applied materials & interfaces. 2012;4:235-43
121. Wang T, Jiang H, Zhao Q, Wang S, Zou M, Cheng G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: effect of silica architecture on immunological properties. Int J Pharm. 2012;436:351-8
122. Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Current Opinion in Chemical Biology. 2005;9:674-9
123. Li H, Fierens K, Zhang Z, Vanparijs N, Schuijs MJ, Van Steendam K. et al. Spontaneous Protein Adsorption on Graphene Oxide Nanosheets Allowing Efficient Intracellular Vaccine Protein Delivery. ACS applied materials & interfaces. 2016;8:1147-55
124. Villa CH, Dao T, Ahearn I, Fehrenbacher N, Casey E, Rey DA. et al. Single-walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano. 2011;5:5300-11
125. Xu L, Xiang J, Liu Y, Xu J, Luo Y, Feng L. et al. Functionalized graphene oxide serves as a novel vaccine nano-adjuvant for robust stimulation of cellular immunity. Nanoscale. 2016;8:3785-95
126. Cambi A, Lidke DS, Arndt-Jovin DJ, Figdor CG, Jovin TM. Ligand-Conjugated Quantum Dots Monitor Antigen Uptake and Processing by Dendritic Cells. Nano Letters. 2007;7:970-7
127. Sen D, Deerinck TJ, Ellisman MH, Parker I, Cahalan MD. Quantum Dots for Tracking Dendritic Cells and Priming an Immune Response In Vitro and In Vivo. PLOS ONE. 2008;3:e3290
128. Xiang J, Xu L, Gong H, Zhu W, Wang C, Xu J. et al. Antigen-Loaded Upconversion Nanoparticles for Dendritic Cell Stimulation, Tracking, and Vaccination in Dendritic Cell-Based Immunotherapy. ACS nano. 2015;9:6401-11
129. Sungsuwan S, Yin ZJ, Huang XF. Lipopeptide-Coated Iron Oxide Nanoparticles as Potential Glycoconjugate-Based Synthetic Anticancer Vaccines. Acs Appl Mater Inter. 2015;7:17535-44
130. Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng. 2007;9:229-56
131. Gonzalez SF, Degn SE, Pitcher LA, Woodruff M, Heesters BA, Carroll MC. Trafficking of B cell antigen in lymph nodes. Annual review of immunology. 2011;29:215-33
132. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-52
133. Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm Res. 1997;14:1568-73
134. Zhang S, Li J, Lykotrafitis G, Bao G, Suresh S. Size-Dependent Endocytosis of Nanoparticles. Advanced materials. 2009;21:419-24
135. Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315-22
136. Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22:2406-12
137. Cruz LJ, Tacken PJ, Rueda F, Domingo JC, Albericio F, Figdor CG. Targeting nanoparticles to dendritic cells for immunotherapy. Methods in enzymology. 2012;509:143-63
138. Cho NH, Cheong TC, Min JH, Wu JH, Lee SJ, Kim D. et al. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nature nanotechnology. 2011;6:675-82
139. Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404-13
140. Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110-4
141. Yukiko Nishioka HY. Lymphatic targeting with nanoparticulate system. Advanced Drug Delivery Reviews. 2001;47:55-64
142. Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112:26-34
143. Hashida M, Egawa M, Muranishi S, Sezaki H. Role of intramuscular administration of water-in-oil emulsions as a method for increasing the delivery of anticancer to regional lymphatics. Journal of Pharmacokinetics and Biopharmaceutics. 1977;5:225-39
144. Nakamoto Y, Hashida M, Muranishi S, Sezaki H. Studies on pharmaceutical modification of anticancer agents. II. Enhanced delivery of bleomycin into lymph by emulsions and drying emulsions. Chemical & pharmaceutical bulletin. 1975;23:3125-31
145. Videira MA, Botelho MF, Santos AC, Gouveia LF, Pedroso de Lima JJ, Almeida AJ. Lymphatic Uptake of Pulmonary Delivered Radiolabelled Solid Lipid Nanoparticles. Journal of Drug Targeting. 2002;10:607-13
146. Paliwal R, Rai S, Vaidya B, Khatri K, Goyal AK, Mishra N. et al. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 2009;5:184-91
147. Maincent P, Thouvenot P, Amicabile C, Hoffman M, Kreuter J, Couvreur P. et al. Lymphatic Targeting of Polymeric Nanoparticles After Intraperitoneal Administration in Rats. Pharmaceutical Research. 1992;9:1534-9
148. Tumer A, Kirby C, Senior J, Gregoriadis G. Fate of Cholesterol-Rich Liposomes after Subcutaneous Injection into Rats. Biochim Biophys Acta. 1983;760:119-25
149. Perezsoler R, Lopezberestein G, Jahns M, Wright K, Kasi LP. Distribution of Radiolabeled Multilamellar Liposomes Injected Intralymphatically and Subcutaneously. Int J Nucl Med Biol. 1985;12:261-6
150. Allen TM, Hansen CB, Guo LSS. Subcutaneous Administration of Liposomes - a Comparison with the Intravenous and Intraperitoneal Routes of Injection. Biochim Biophys Acta. 1993;1150:9-16
151. Oussoren C, Zuidema J, Crommelin DJ, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid compostion and lipid dose. Biochim Biophys Acta. 1997;1328:261-72
152. Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Advanced drug delivery reviews. 2001;50:143-56
153. Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual review of immunology. 1999;17:593-623
154. Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P. et al. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15:1403-9
155. Chen H-W, Huang C-Y, Lin S-Y, Fang Z-S, Hsu C-H, Lin J-C. et al. Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials. 2016;106:111-8
156. Mbow ML, De Gregorio E, Ulmer JB. Alum's adjuvant action: grease is the word. Nat Med. 2011;17:415-6
157. Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597-608
158. Nuhn L, Vanparijs N, De Beuckelaer A, Lybaert L, Verstraete G, Deswarte K. et al. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc Natl Acad Sci U S A. 2016;113:8098-103
159. Ilyinskii PO, Roy CJ, O'Neil CP, Browning EA, Pittet LA, Altreuter DH. et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine. 2014;32:2882-95
160. Kim D, Kwon HJ, Lee Y. Activation of Toll-like receptor 9 and production of epitope specific antibody by liposome-encapsulated CpG-DNA. Bmb Rep. 2011;44:607-12
161. Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35:814-24
162. Crouse J, Kalinke U, Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nature reviews Immunology. 2015;15:231-42
163. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical microbiology reviews. 2009;22:240-73
164. Liu Q, Jia JL, Yang TY, Fan QZ, Wang LY, Ma GH. Pathogen-Mimicking Polymeric Nanoparticles based on Dopamine Polymerization as Vaccines Adjuvants Induce Robust Humoral and Cellular Immune Responses. Small. 2016;12:1744-57
165. Kreutz M, Giquel B, Hu Q, Abuknesha R, Uematsu S, Akira S. et al. Antibody-Antigen-Adjuvant Conjugates Enable Co-Delivery of Antigen and Adjuvant to Dendritic Cells in Cis but Only Have Partial Targeting Specificity. Plos One. 2012;7:e40208
166. Jegerlehner A, Storni T, Lipowsky G, Schmid M, Pumpens P, Bachmann MF. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur J Immunol. 2002;32:3305-14
167. Bachmann MF, Kalinke U, Althage A, Freer G, Burkhart C, Roost H-P. et al. The Role of Antibody Concentration and Avidity in Antiviral Protection. Science. 1997;276:2024-7
168. Bachmann MF, Zinkernagel RM. The influence of virus structure on antibody responses and virus serotype formation. Immunology today. 1996;17:553-8
169. Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annual review of immunology. 1997;15:235-70
170. Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of Autoantibody Induction by Conjugated Papillomavirus Virus-Like Particles. The Journal of Immunology. 2002;169:6120-6
171. Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448-51
172. Chackerian B, Lowy DR, Schiller JT. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. Journal of Clinical Investigation. 2001;108:415-23
173. Chackerian B, Lowy DR, Schiller JT. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proceedings of the National Academy of Sciences. 1999;96:2373-8
174. Barrington R, Zhang M, Fischer M, Carroll MC. The role of complement in inflammation and adaptive immunity. Immunological reviews. 2001;180:5-15
175. Carter RH, Myers R. Germinal Center Structure and Function: Lessons form CD19. Seminars in immunology. 2008;20:43-8
176. Erik B. Puffer JKP, Jessica J. Hollenbeck, John A. Kink and Laura L. Kiessling. Activating B Cell Signaling with Defined Multivalent Ligands. ACS Chem Bio. 2006;2:252-262
177. Zinkernagel RM. On natural and artificial vaccinations. Annual review of immunology. 2003;21:515-46
178. Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annual review of immunology. 2010;28:157-83
179. Smith DM, Simon JK, Baker JR Jr. Applications of nanotechnology for immunology. Nature reviews Immunology. 2013;13:592-605
180. Little SR. Reorienting our view of particle-based adjuvants for subunit vaccines. P Natl Acad Sci USA. 2012;109:999-1000
181. Hu CJ, Chien CY, Liu MT, Fang ZS, Chang SY, Juang RH. et al. Multi-antigen avian influenza a (H7N9) virus-like particles: particulate characterizations and immunogenicity evaluation in murine and avian models. BMC Biotechnol. 2017;17:2
182. Naskalska A, Pyrc K. Virus Like Particles as Immunogens and Universal Nanocarriers. Pol J Microbiol. 2015;64:3-13
183. Slütter B, Soema PC, Ding Z, Verheul R, Hennink W, Jiskoot W. Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. Journal of Controlled Release. 2010;143:207-14
184. Jones DS, Rowe CG, Chen B, Reiter K, Rausch KM, Narum DL. et al. A Method for Producing Protein Nanoparticles with Applications in Vaccines. PLoS ONE. 2016;11:e0138761
185. Milley B, Kiwan R, Ott GS, Calacsan C, Kachura M, Campbell JD. et al. Optimization, Production, and Characterization of a CpG-Oligonucleotide-Ficoll Conjugate Nanoparticle Adjuvant for Enhanced Immunogenicity of Anthrax Protective Antigen. Bioconjug Chem. 2016;27:1293-304
186. Hanson MC, Abraham W, Crespo MP, Chen SH, Liu H, Szeto GL. et al. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine. 2015;33:861-8
187. Alberto J, Salazar-Gonzalez, Gonzalez-Ortega O, Rosales-Mendoza S. Gold nanoparticles and vaccine development. Expert Rev Vaccines. 2015;14:1197-211
188. Ding Y, Jiang ZW, Saha K, Kim CS, Kim ST, Landis RF. et al. Gold Nanoparticles for Nucleic Acid Delivery. Mol Ther. 2014;22:1075-83
189. Jin RC, Wu GS, Li Z, Mirkin CA, Schatz GC. What controls the melting properties of DNA-linked gold nanoparticle assemblies? J Am Chem Soc. 2003;125:1643-54
190. Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607-9
191. Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027-30
192. Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, Mirkin CA. Regulating Immune Response Using Polyvalent Nucleic Acid-Gold Nanoparticle Conjugates. Mol Pharmaceut. 2009;6:1934-40
193. Tao WQ, Gill HS. M2e-immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine. 2015;33:2307-15
194. Tao WQ, Ziemer KS, Gill HS. Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine-Uk. 2014;9:237-52
195. Totaro KA, Liao X, Bhattacharya K, Finneman JI, Sperry JB, Massa MA. et al. Systematic Investigation of EDC/sNHS-Mediated Bioconjugation Reactions for Carboxylated Peptide Substrates. Bioconjugate Chemistry. 2016;27:994-1004
196. Handke N, Ficheux D, Rollet M, Delair T, Mabrouk K, Bertin D. et al. Lysine-tagged peptide coupling onto polylactide nanoparticles coated with activated ester-based amphiphilic copolymer: a route to highly peptide-functionalized biodegradable carriers. Colloids and surfaces B, Biointerfaces. 2013;103:298-303
197. Heuking S, Iannitelli A, Di Stefano A, Borchard G. Toll-like receptor-2 agonist functionalized biopolymer for mucosal vaccination. Int J Pharm. 2009;381:97-105
198. Lee H, Lee Y, Statz AR, Rho J, Park TG, Messersmith PB. Substrate-Independent Layer-by-Layer Assembly by Using Mussel-Adhesive-Inspired Polymers. Advanced materials. 2008;20:1619-23
199. Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426-30
200. Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. P Natl Acad Sci USA. 2006;103:12999-3003
201. Madhurakkat Perikamana SK, Lee J, Lee YB, Shin YM, Lee EJ, Mikos AG. et al. Materials from Mussel-Inspired Chemistry for Cell and Tissue Engineering Applications. Biomacromolecules. 2015;16:2541-55
202. Lee BP, Messersmith PB, Israelachvili JN, Waite JH. Mussel-Inspired Adhesives and Coatings. Annu Rev Mater Res. 2011;41:99-132
203. Qin L, Li X, Kang S-Z, Mu J. Gold nanoparticles conjugated dopamine as sensing platform for SERS detection. Colloids and Surfaces B: Biointerfaces. 2015;126:210-6
204. Hu Q, Wu M, Fang C, Cheng C, Zhao M, Fang W. et al. Engineering Nanoparticle-Coated Bacteria as Oral DNA Vaccines for Cancer Immunotherapy. Nano Letters. 2015;15:2732-9
205. Lee SY, Cho H-J. Dopamine-conjugated poly(lactic-co-glycolic acid) nanoparticles for protein delivery to macrophages. Journal of Colloid and Interface Science. 2017;490:391-400
206. Chen WH, Huang L. Induction of cytotoxic T-lymphocytes and antitumor activity by a liposomal lipopeptide vaccine. Molecular pharmaceutics. 2008;5:464-71
207. Rossman JS, Leser GP, Lamb RA. Filamentous Influenza Virus Enters Cells via Macropinocytosis. Journal of virology. 2012;86:10950-60
208. Shaikh FY, Utley TJ, Craven RE, Rogers MC, Lapierre LA, Goldenring JR. et al. Respiratory Syncytial Virus Assembles into Structured Filamentous Virion Particles Independently of Host Cytoskeleton and Related Proteins. PloS one. 2012:7
209. Galloway AL, Murphy A, DeSimone JM, Di J, Herrmann JP, Hunter ME. et al. Development of a nanoparticle-based influenza vaccine using the PRINT technology. Nanomedicine. 2013;9:523-31
210. Dykman LA, Sumaroka MV, Staroverov SA, Zaitseva IS, Bogatyrev VA. Immunogenic properties of colloidal gold. Biol Bull. 2004;31:75-9
211. Behera T, Swain P. Antigen adsorbed calcium phosphate nanoparticles stimulate both innate and adaptive immune response in fish, Labeo rohita H. Cellular immunology. 2011;271:350-9
212. Amini Y, Moradi B, Tafaghodi M, Meshkat Z, Ghazvini K, Fasihi-Ramandi M. TB trifusion antigen adsorbed on calcium phosphate nanoparticles stimulates strong cellular immunity in mice. Biotechnol Bioproc E. 2016;21:653-8
213. Li X, Aldayel AM, Cui Z. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. Journal of controlled release. 2014;173:148-57
214. Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green MLH, Sim RB. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43:193-201
215. Li H, Fierens K, Zhang Z, Vanparijs N, Schuijs MJ, Van Steendam K. et al. Spontaneous Protein Adsorption on Graphene Oxide Nanosheets Allowing Efficient Intracellular Vaccine Protein Delivery. ACS Appl Mater Interfaces. 2016;8:1147-55
216. Mody KT, Popat A, Mahony D, Cavallaro AS, Yu CZ, Mitter N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale. 2013;5:5167-79
217. Castaldello A, Brocca-Cofano E, Voltan R, Triulzi C, Altavilla G, Laus M. et al. DNA prime and protein boost immunization with innovative polymeric cationic core-shell nanoparticles elicits broad immune responses and strongly enhance cellular responses of HIV-1 tat DNA vaccination. Vaccine. 2006;24:5655-69
218. Nagamoto T, Hattori Y, Takayama K, Maitani Y. Novel chitosan particles and chitosan-coated emulsions inducing immune response via intranasal vaccine delivery. Pharm Res. 2004;21:671-4
219. Singh M, Briones M, Ott G, O'Hagan D. Cationic microparticles: A potent delivery system for DNA vaccines. Proceedings of the National Academy of Sciences. 2000;97:811-6
220. O'Hagan D, Singh M, Ugozzoli M, Wild C, Barnett S, Chen M. et al. Induction of potent immune responses by cationic microparticles with adsorbed human immunodeficiency virus DNA vaccines. Journal of virology. 2001;75:9037-43
221. Singh M, Ott G, Kazzaz J, Ugozzoli M, Briones M, Donnelly J. et al. Cationic Microparticles Are an Effective Delivery System for Immune Stimulatory CpG DNA. Pharmaceutical Research. 2001;18:1476-9
222. Singh M, Kazzaz J, Chesko J, Soenawan E, Ugozzoli M, Giuliani M. et al. Anionic microparticles are a potent delivery system for recombinant antigens from Neisseria meningitidis serotype B. Journal of pharmaceutical sciences. 2004;93:273-82
223. Jung T, Breitenbach A, Kissel T. Sulfobutylated poly(vinyl alcohol)-graft-poly(lactide-co-glycolide)s facilitate the preparation of small negatively charged biodegradable nanospheres. Journal of Controlled Release. 2000;67:157-69
224. Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nature nanotechnology. 2012;7:779-86
225. Sivaraman B, Fears KP, Latour RA. Investigation of the Effects of Surface Chemistry and Solution Concentration on the Conformation of Adsorbed Proteins Using an Improved Circular Dichroism Method. Langmuir. 2009;25:3050-6
226. Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980-5
227. Hu CJ, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118-21
228. Hu CM, Fang RH, Luk BT, Chen KN, Carpenter C, Gao W. et al. 'Marker-of-self' functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale. 2013;5:2664-8
229. Weissenhorn W, Dessen A, Calder LJ, Harrison SC, Skehel JJ, Wiley DC. Structural basis for membrane fusion by enveloped viruses. Mol Membr Biol. 1999;16:3-9
230. Hu CM, Fang RH, Luk BT, Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nature nanotechnology. 2013;8:933-8
231. Wang F, Fang RH, Luk BT, Hu CMJ, Thamphiwatana S, Dehaini D. et al. Nanoparticle-Based Antivirulence Vaccine for the Management of Methicillin-Resistant Staphylococcus aureus Skin Infection. Adv Funct Mater. 2016;26:1628-35
232. Fang RH, Hu CMJ, Luk BT, Gao WW, Copp JA, Tai YY. et al. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014;14:2181-8
233. Lerm M, Schmidt G, Aktories K. Bacterial protein toxins targeting Rho GTPases. Fems Microbiol Lett. 2000;188:1-6
Author Biography
 Saborni Chattopadhyay is a PhD candidate in the Institute of Biomedical Sciences at Academia Sinica, Taiwan. She received her M. Tech in Biological Sciences and Bioengineering from Indian Institute of Technology Kanpur. Her research interests include cancer, therapeutics and drug delivery. Saborni is an avid reader and enjoys travelling.
Saborni Chattopadhyay is a PhD candidate in the Institute of Biomedical Sciences at Academia Sinica, Taiwan. She received her M. Tech in Biological Sciences and Bioengineering from Indian Institute of Technology Kanpur. Her research interests include cancer, therapeutics and drug delivery. Saborni is an avid reader and enjoys travelling.
 Jui-Yi Chen is a research assistant in the Institute of Biomedical Sciences at Academia Sinica, Taiwan. She received her master's degree in Material Science and Engineering from the National Tsing-Hua University in Taiwan. Her research interests include biomimetic materials and drug delivery.
Jui-Yi Chen is a research assistant in the Institute of Biomedical Sciences at Academia Sinica, Taiwan. She received her master's degree in Material Science and Engineering from the National Tsing-Hua University in Taiwan. Her research interests include biomimetic materials and drug delivery.
 Dr. Hui-Wen Chen is an Assistant Professor in the Department of Veterinary Medicine at the National Taiwan University. She received her Ph.D. in School of Veterinary Medicine from the National Taiwan University. She is also an officially accredited veterinarian in Taiwan. Her research interests include the viral epidemiology, viral immunity, and development of viral vaccines.
Dr. Hui-Wen Chen is an Assistant Professor in the Department of Veterinary Medicine at the National Taiwan University. She received her Ph.D. in School of Veterinary Medicine from the National Taiwan University. She is also an officially accredited veterinarian in Taiwan. Her research interests include the viral epidemiology, viral immunity, and development of viral vaccines.
 Dr. Che-Ming Jack Hu is an Assistant Research Fellow in the Institute of Biomedical Sciences at Academia Sinica, Taiwan. He received his Ph.D. in Biomedical Engineering from the University of California, San Diego. The current research interests of Dr. Hu's research group include biomimetic materials and nanoparticles for drug delivery and vaccine applications.
Dr. Che-Ming Jack Hu is an Assistant Research Fellow in the Institute of Biomedical Sciences at Academia Sinica, Taiwan. He received his Ph.D. in Biomedical Engineering from the University of California, San Diego. The current research interests of Dr. Hu's research group include biomimetic materials and nanoparticles for drug delivery and vaccine applications.
![]() Corresponding author: Email: chusinica.edu.tw
Corresponding author: Email: chusinica.edu.tw

 Global reach, higher impact
Global reach, higher impact