ISSN: 2206-7418
Nanotheranostics 2017; 1(4):440-449. doi:10.7150/ntno.21905 This issue Cite
Research Paper
Ultrasound-Stimulated Drug Delivery Using Therapeutic Reconstituted High-Density Lipoprotein Nanoparticles
1. Department of Bioengineering, University of Texas at Dallas, Richardson, TX 75080 USA.
2. Department of Medical Ultrasound, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
3. Department of Pediatrics, University of North Texas Health Sciences Center, Fort Worth TX 76107 USA;
4. Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX 75390 USA.
Abstract
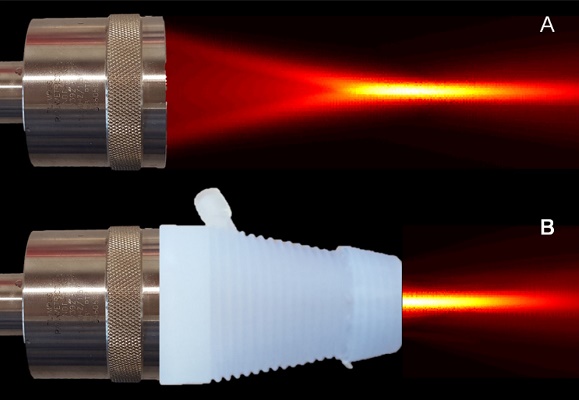
The abnormal tumor vasculature and the resulting abnormal microenvironment are major barriers to optimal chemotherapeutic drug delivery. It is well known that ultrasound (US) can increase the permeability of the tumor vessel walls and enhance the accumulation of anticancer agents. Reconstituted high-density lipoproteins (rHDL) nanoparticles (NPs) allow selective delivery of anticancer agents to tumor cells via their overexpressed scavenger receptor type B1 (SR-B1) receptor. The goal of this study is to investigate the potential of noninvasive US therapy to further improve delivery and tumor uptake of the payload from rHDL NPs, preloaded with an infrared dye (IR-780), aimed to establish a surrogate chemotherapeutic model with optical localization. Athymic nude mice were implanted orthotopically with one million breast cancer cells (MDA-MB-231/Luc). Three weeks later, animals were divided into seven groups with comparable mean tumor size: control, low, moderate, and high concentration of rHDL NPs alone groups, as well as these three levels of rHDL NPs plus US therapy groups (N = 7 to 12 animals per group), where low, moderate and high denote 5, 10, and 50 µg of the IR-780 dye payload per rHDL NP injection, respectively. The US therapy system included a single element focused transducer connected in series with a function generator and power amplifier. A custom 3D printed cone with an acoustically transparent aperture and filled with degassed water allowed delivery of focused US energy to the tumor tissue. US exposure involved a pulsed sequence applied for a duration of 5 min. Each animal in the US therapy groups received a slow bolus co-injection of MB contrast agent and rHDL NPs. Animals were imaged using a whole-body optical system to quantify intratumoral rHDL NP accumulation at baseline and again at 1 min, 30 min, 24 h, and 48 h. At 48 h, all animals were euthanized and tumors were excised for ex vivo analysis. We investigated a noninvasive optical imaging method for monitoring the effects of US-stimulated drug delivery of IR-780 dye-loaded rHDL NPs in living animals. No change in optical imaging data was found in the control animals. However, there was considerable dye accumulation (surrogate drug) within 48 h in the low (5 µg), moderate (10 µg), and high (50 µg) rHDL NP concentration-dosed group animals (p < 0.09). With US therapy added to the experimental protocol, there was an additional and significant increase in local tumor drug uptake at 48 h (p < 0.02). Optical image data collected from ex vivo tumor samples confirmed tumor retention of the IR-780 dye-loaded rHDL NPs and correlated positively with in vivo optical imaging results (R2 > 0.69, p < 0.003). IR-780 dye extraction from the tumor tissue samples confirmed the in vivo and ex vivo US therapy findings. Overall, the addition of US therapy considerably improved local rHDL NP accumulation in tumor tissue. This study concludes that US-mediated drug delivery can facilitate tumor uptake of rHDL NPs and more research is warranted to optimize the drug dosing schedule and the respective therapeutic protocols.
Keywords: cancer, drug delivery, microbubble contrast agents, nanoparticles, ultrasound.

 Global reach, higher impact
Global reach, higher impact